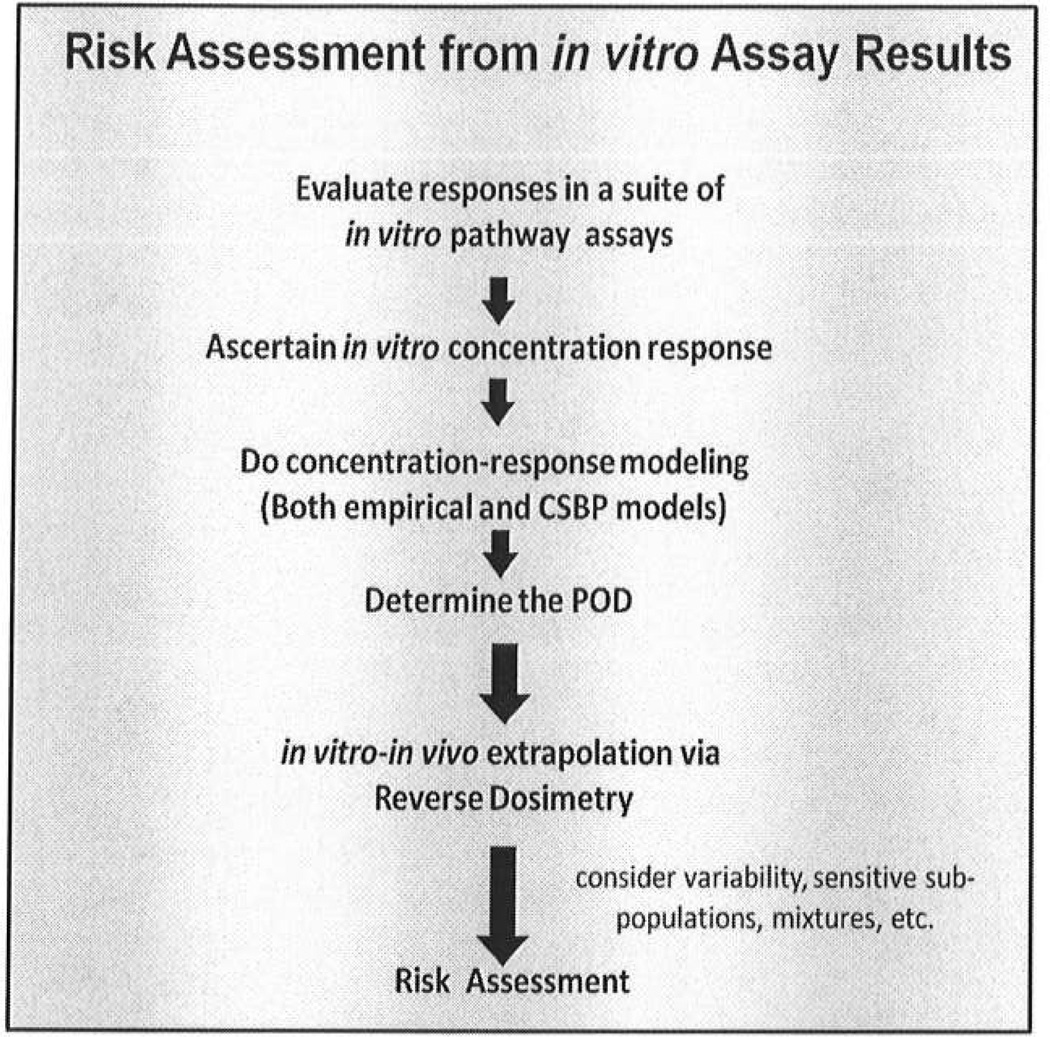
Figure 2. A Schematic showing steps in a toxicity pathway-based risk assessment.
Results from the panel of assays identify the pathway targets and generate a point of departure (POD) for the subsequent risk assessment as an in vitro concentration. Computational systems biology pathway (CSBP) modeling of circuitry and dynamics for the assay system indicates the expected shape of the dose response at lower doses, leading to a POD. The POD concentration is then converted to an exposure standard through techniques of reverse dosimetry implemented by pharmacokinetic modeling. This step takes advantages of in vitro-in vivo extrapolation (IVIVE).