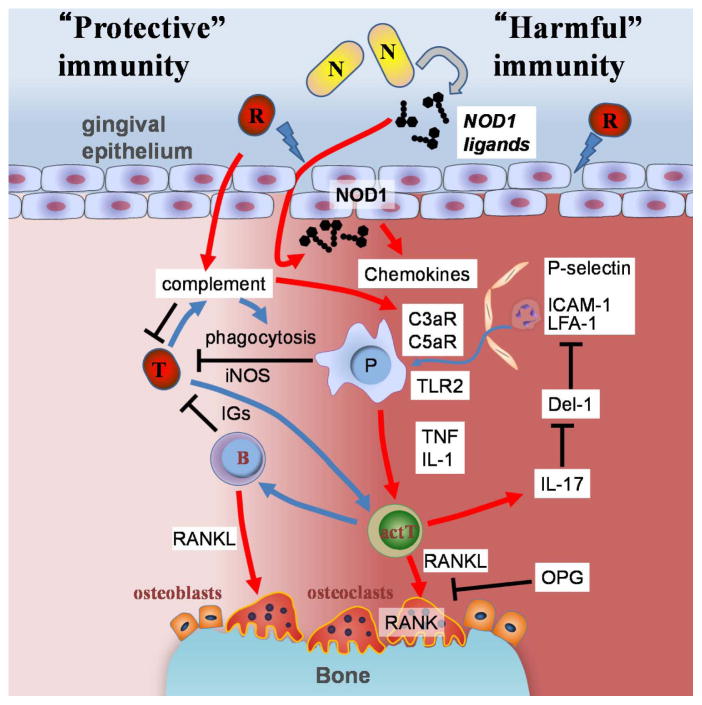
Figure 1. Models for host protective and damaging immune responses to oral bacteria in periodontitis.
Alveolar bone loss in periodontitis is primarily associated with immune responses to oral bacteria. The immune responses provide protection against translocated bacteria (T) but also mediate alveolar bone resorption which is harmful to host tissues. Host protective immune responses include elimination of pathogens by phagocytic cells (P) and production of antigen-specific immunoglobulin (IGs) which are mediated by antigen presenting phagocytic cells and activated T cells (actT). Inducible NO synthase (iNOS) is also known to be involved in host protective immune responses. The number of bone absorbing osteoclasts increase at sites below the damaged gingiva during periodontitis development. In the model, host damaging red complex bacteria (R) damage the epithelial barrier function. The epithelial damage allows the translocation of harmful bacteria or immunostimulatory bacterial components into the gingival tissue. NOD1 ligands are released from particular types of oral bacteria (N) which induces recruitment of phagocytotic cells to the damaged gingiva via induction of chemokines such as CXCLs and CCLs (e.g. IL-8 and CCL3, respectively). Translocated bacteria (T) are eliminated by phagocytosis and other mechanisms through recruited immune cells, bacterial opsonization, and complement activation. A red complex bacterium such as P. gingivalis possesses the ability to process complement factors. The processed complement factors further induce recruitment of myelomonocytic cells to the lesion. IL-17 inhibits expression of Del-1 that interferes with neutrophil adhesion and molecule-dependent neutrophil recruitment. Some myelomonocytic cells are major sources of immunostimulatory molecules which facilitate the development of secondary immune responses to the bacteria. Immunization against P. gingivalis is shown to protect hosts from alveolar bone loss. Myelomonocytic cells also release bone loss-inducing inflammatory cytokines, TNF and IL-1, upon bacterial stimulation of PRRs such as TLR2. RANKL is expressed on activated T, B cells and osteoblasts that are stimulated by these inflammatory cytokines, T cells, and is important for alveolar bone loss in experimental periodontitis models. Therefore, protective immune responses that are activated to eliminate translocated bacteria can also damage the host by the induction of alveolar bone loss.