Table 1.
Synthesis of 2-methyl-4-(3-aminophenyl)-3-butyn-2-ol-from 3-bomoaniline and 2-methyl-3-butyn-2-ol.a
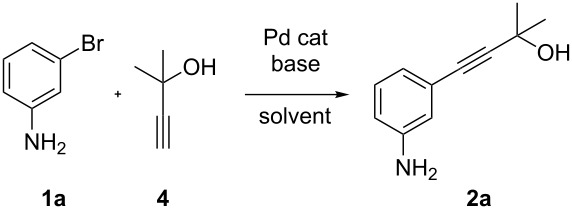 | ||||
| Entry | Solvent | Base | Ligand | Yield (%) |
| 1 | toluene | TBAF | PPh3 | 32 |
| 2 | THF | TBAF | PPh3 | 61 |
| 3 | DMF | TBAF | PPh3 | 9 |
| 4 | THF | K2CO3 | PPh3 | 14 |
| 5 | THF | NEt3 | PPh3 | 5 |
| 6 | THF | piperidine | PPh3 | 17 |
| 7 | THF | DBU | PPh3 | 84 |
| 8 | THF | DBU | P(p-FC6H4)3 | 75 |
| 9 | THF | DBU | P(p-tol)3 | 89 |
| 10 | THF | DBU | P(o-tol)3 | 45 |
| 11 | THF | DBU | dppe | 50 |
aReaction conditions: 1a (1.0 mmol), 4 (1.2 mmol), Pd(OAc)2 (0.03 mmol), ligand (6 mol %), base (3 equiv), 6 h, 80 °C. Yields were determined by GC using tetradecane as an internal standard.
