Abstract
Objective
To determine if central visual loss is associated with driving cessation, driving restriction or other-driver preference.
Design
Cross-sectional study
Participants
Sixty four subjects with either bilateral (<20/32 in better eye) or severe unilateral visual loss (<20/200) due to age related macular degeneration (AMD) and 58 normally-sighted controls between 60 and 80 years of age.
Methods
Participants self-reported their driving habits using a standardized questionnaire. Other-driver preference was defined as preferring that another drive when there is more than 1 driver in the car. Subjects reporting 2 or more driving limitations (out of a list of nine limitations) were considered to have restricted their driving.
Main Outcome Measures
Self-reported driving cessation, other-driver preference, and driving restriction.
Results
AMD subjects were older (74.7 vs. 69.7 years), had worse visual acuity (VA) (mean better eye logarithm of minimal angle of resolution (logMAR) VA = 0.43 vs. 0.08) and contrast sensitivity (CS) (1.4 vs. 1.9 logCS units) and were more likely to be white when compared to controls (p<0.001 for all). Drivers in the group with AMD-related vision loss were more likely to avoid driving over longer distances (p<0.001), beyond 1 hour (p=0.03), at night (p=0.005) and in unfamiliar conditions (p=0.001). In multivariable models, driving cessation was associated with worse better-eye VA (odds ratio (OR) =1.5 per one-line decrement in VA; p<0.001) and worse binocular CS (OR=1.36 per 0.1 log CS increment, p=0.005), however; AMD group status was not associated with driving cessation (OR=1.9, p=0.35). Factors predictive of driving restriction were AMD group status (OR=9.0, p=0.004), worse vision (OR=2.5 per line VA loss, p<0.001), lower CS (OR=2.2 per 0.1logCS increment, p<0.001) and female gender (OR=27.9, p=0.002). Other-driver preference was more common with worse vision (OR=1.6 per 0.1 logMAR increment, p=0.003), female gender (OR=4.5, p=0.02) and being married (OR=3.8, p=0.04).
Conclusions
Most patients with AMD-related central vision loss continue to drive but demonstrate significant driving restrictions. Subjects with more advanced loss of visual acuity and contrast sensitivity are more likely to experience driving restrictions. More work is required to determine which driving adaptations adopted by visually-impaired AMD patients best balance safety and independence.
Introduction
Age related macular degeneration (AMD) is the most frequent cause of severe, irreversible visual impairment in the developed world.1,2 In the United States alone there are an estimated 1.75 million adults with neovascular AMD or geographic atrophy, and these cases account for approximately 46% of cases of severe visual loss.3,4 With improved life expectancies and an aging population, the global prevalence of AMD will further rise in the years to come. AMD predominantly leads to loss of central vision and is rarely associated with systemic disease (unlike other common diseases such as cataract or diabetes). Additionally, subjects with AMD have well-preserved peripheral vision. Hence, AMD is a good model disease for assessing the effects of central vision loss on important patient outcomes. Prevention of AMD-related vision loss and proper rehabilitation and counseling of individuals with untreatable AMD-related vision loss will become increasingly important, and rehabilitation strategies for visually- impaired AMD patients will serve as a model to guide the care of other patients with primarily central vision loss.
Several important functional domains are affected in visually impaired AMD patients, including mobility,5 reading, recognizing faces, watching TV and driving.6 Of these, driving has a particularly strong association with quality of life, especially in the United States where public transportation is frequently limited or not available. Driving offers independence to older individuals and limitation or cessation of driving is reported to be associated with incident depression, increased dependence, reduced accessibility to health care and higher mortality.7,8,9 Psychometric tools such as the 25-item National Eye Institute Visual Function Questionnaire (NEI-VFQ) have a limited number of questions about driving which have been used to characterize driving impairment on quality of life in patients with AMD,10, 11 and have been used to demonstrate that AMD treatments can prevent driving cessation and improve perceived driving ability.12 However the small number of driving-related NEI-VFQ questions precludes a comprehensive description of the types of driving alterations which occur in conjunction with AMD-related vision loss. In particular, there is very little research describing how, and how often, individuals with vision loss due to AMD restrict their driving,13 and literature from other eye diseases suggests that restriction of driving in particular settings may be a more frequent adaptation to vision loss than driving cessation.14
Here, we use a detailed driving questionnaire to compare the driving habits and driving adaptations found in a sample of AMD patients with vision loss and normally-sighted controls. Evaluated adaptations to driving included complete cessation of driving, restriction of driving to more favorable conditions/locations, and preferring that another person drive, each of which has its own psychosocial impact.9
Methods
Study participants
The study was approved by the Johns Hopkins Medical Institutions’ Institutional Review Board and written informed consent was obtained from all study subjects. Participants were recruited from a convenience sample of patients being cared for at the Wilmer Eye Institute at Johns Hopkins. Subjects’ charts were prescreened for eligibility, and eligible subjects had to be between the ages of 60–80 years and had to be former or current drivers. Patients were recruited between July 2009 and June 2012.
AMD subjects were required to have bilateral AMD with evidence of drusen, geographic atrophy, or choroidal neovascularization in both eyes. Visual acuity (VA) was required to be 20/32 or worse in both eyes, or worse than 20/200 in one eye regardless of the second-eye vision. Subjects with deterioration of visual angle by greater than 50% over past 3 months and those who received intravitreal injections over the past month were excluded. Controls subjects were glaucoma suspects or ocular hypertensives being treated at the same eye hospital as the cases with a VA of 20/40 or better and normal visual fields.15 Participants also had to be residing in the mid-Atlantic region (defined for this Baltimore-based study as Maryland, Virginia, Delaware, the District of Columbia and Pennsylvania). Glaucoma suspects were chosen as a comparison group as they: (1) would be most likely to have similar social and health/behavior characteristics as the AMD patients seeking care at the same hospital, and (2) have been shown to be similar with regards to visual acuity, contrast sensitivity (CS), and visual fields to older individuals without eye disease characterized in previous population based surveys.15
Evaluation of Driving Habits
Driving habits were evaluated with an interviewer-administered questionnaire taken from the Salisbury Eye Evaluation Driving Study (SEEDS), which extends upon questionnaires previously used in the Salisbury Eye Evaluation.16,17 Subjects were asked, “Have you driven a car in the past three months?” to assess driving cessation. In those who were currently driving, 9 different driving limitations were assessed: (1) not driving outside of the mid-Atlantic region, (2) not driving more than one hour away from home, (3) not driving to neighboring towns or areas, (4) not driving beyond the neighborhood, (5) not driving in the rain, (6) not driving at night, (7) not driving in unfamiliar areas, (8) driving less than twice per week, and (9) driving less than 5,000 miles per year (the standard for restricted driving in Maryland). Each of these limitations was assessed for the past year except for driving at night and driving in unfamiliar areas, which were assessed for the past three months. The questionnaire asked whether a person had performed a particular driving activity at all during the appointed time frame, but not if they chose to avoid that particular driving activity or if they were legally prohibited from doing it.
Driver preference was assessed by asking subjects, “in a typical week when you travel in a car, how often are you the driver?” Subjects who reported that they were the driver 50% or less of the time they rode in a car were considered to have other-driver preference.
Measurement of Vision and Covariates
Monocular VA was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart and better-eye VA was converted to the logarithm of the minimum angle of resolution (logMAR) for use in statistical analysis. Binocular CS was measured using the Pelli-Robson chart at 1 meter with subjects wearing their usual correction, and was converted into log units (log CS) for analysis. Both eyes were examined after pupillary dilation for significant lenticular changes in phakic eyes using the Wilmer cataract grading system, or posterior capsular opacification (PCO) in pseudophakic eyes, as previously described.18,19
Demographic information was collected by self-report, including age, gender, race, employment status, years of education completed, living situation (if the subject lives with any other adults), and marital status. Cognitive ability was assessed using the Mini Mental Status Exam (MMSE) for the Visually Impaired.20 Depressive symptoms were detected using the Geriatric Depression Scale Short Form, with subjects demonstrating 6 or more positive responses considered to have depressive symptoms.21 Medical comorbidities were assessed using a standardized structured medical history questionnaire and summarized as the number of comorbid conditions present.22 Specific comorbidities inquired about included arthritis, broken or fractured hip, back problems, heart attack/myocardial infarction, angina/chest pain, congestive heart failure, peripheral vascular disease, hypertension, diabetes, emphysema, asthma, stroke, Parkinson’s, cancer (other than skin cancer), and vertigo/Meniere’s.
Statistical Analysis
The current study was a secondary analysis from a study powered to detect differences in physical activity and travel patterns in subjects with normal vision, visual field loss from glaucoma, and decreased visual acuity from AMD. The proposed sample size of 60 normally- sighted subjects and 60 visually-impaired subjects with AMD had a greater than 70% power to detect a three-fold higher odds of driving cessation or driving restriction in our AMD patients as compared to controls assuming a type I error probability of 0.05 and a baseline level of driving cessation/driving restriction similar to that observed in prior population-based surveys).15
Group differences for continuous variables were evaluated using the Student t-test or Wilcoxon rank-sum test. Chi-square analysis was used to assess differences in categorical variables. Binary outcomes, including driving cessation, driver preference, and the presence of individual driving limitations were assessed using univariate and multivariable logistic regression models. The number of driving limitations was analyzed as a continuous variable and also converted into a binary variable labeled “driving restriction” and classified as present if 2 or more driving limitations were report.15 Covariates included in multivariable regression models included those with a p value <0.05 on univariate analysis and those demonstrated to have association with driving habits in previous research.15 The statistical analysis was completed using Stata (I/C 12.0, College Station, TX) statistical software package.
RESULTS
Sixty four patients with bilateral AMD and 58 controls were enrolled in the study. Baseline demographics and visual parameters in the two groups are shown in Table 1. AMD patients were older, had worse VA and CS, and were more likely to be white when compared to controls (p≤0.01 for all). In the better seeing eye, 30 subjects (47%) with AMD had geographic atrophy and 34 (53%) had a predominantly scarred neovascular membrane involving the center of the fovea.
Table 1.
Characteristics of study participants in AMD group V/s Controls.
| Parameter evaluated | Controls (n = 58) | AMD (n = 64) | P Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 69.8 (5.29) | 74.8 (4.98) | <0.001 |
| Female gender (%) | 60.3 | 57.1 | 0.72 |
| White race (%) | 77.6 | 93.7 | 0.01 |
| Education (years) | 15.5 (2.2) | 15 (1.9) | 0.13 |
| Unemployment (%) | 60.3 | 79.3 | 0.02 |
| Lives with others (%) | 25.9 | 17.5 | 0.26 |
| Married (%) | 67.2 | 68.3 | 0.11 |
| Health/cognition | |||
| MMSE-VI score | 20.8 (1.4) | 20.6 (1.7) | 0.76 |
| Comorbid illnesses (#) | 2 (2, 3) | 2.5 (1, 3) | 0.54 |
| Depressive symptoms (%) | 5.2 | 4.8 | 0.9 |
| Vision parameters | |||
| Binocular CS, log units | 1.9 (0.12) | 1.4 (0.33) | <0.001 |
| Better eye acuity, logMAR | 0.08 (0.12) | 0.43 (0.32) | <0.001 |
| Worse eye acuity, logMAR | 0.18 (0.14) | 1.05 (0.46) | <0.001 |
| Sig. cataract/PCO, right eye (%) | 16.7 | 27.6 | 0.16 |
| Sig. cataract/PCO, left eyes (%) | 14.8 | 23.3 | 0.25 |
Continuous variables reported as mean (95% CI).AMD = Age related macular degeneration, logMAR = logarithm of the minimum angle of resolution; CS = contrast sensitivity; PCO=Posterior capsular opacification; MMSE VI = Mini-Mental Status
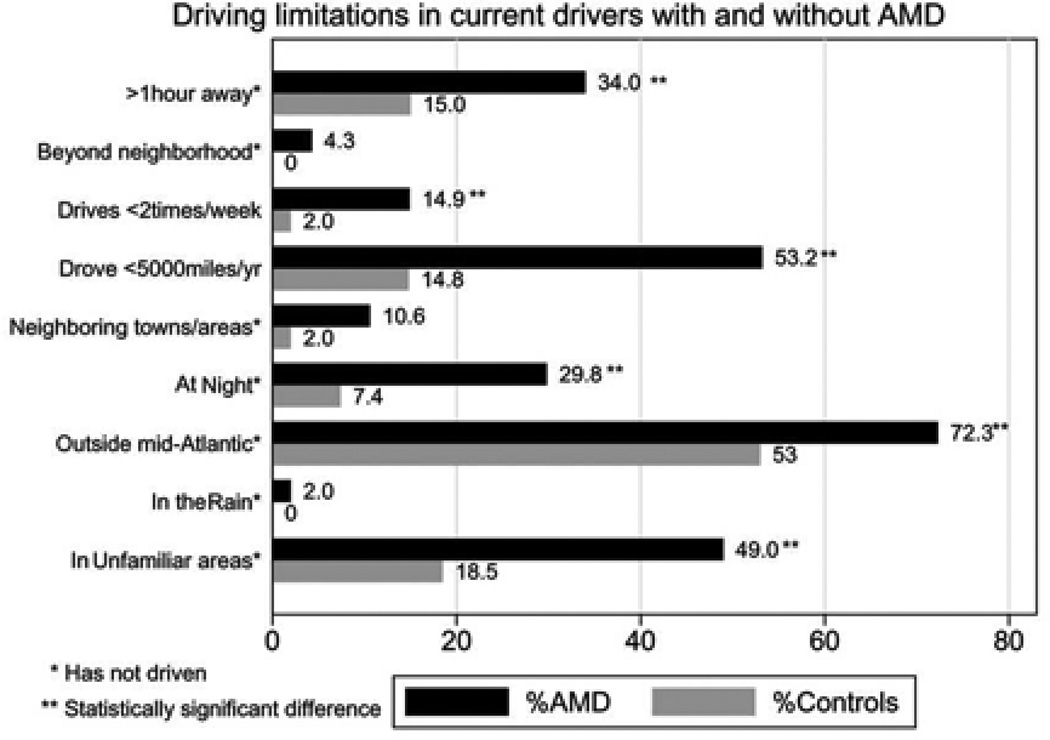
In univariate analyses, more patients in the AMD group had ceased driving as compared to controls (25.4% vs. 6.9%, p=0.006). Subjects who had stopped driving had significantly worse vision in the better seeing eye (logMAR visual acuity = 0.77 vs. 0.08, p=0.001) and CS (log CS = 1.8 vs. 1.2, p=0.03) compared to those who continued to drive. Drivers in the AMD group also had a greater mean number of driving limitations than control group drivers (2.7 vs. 1.1, p<0.001), and were more likely to report two or more driving restrictions (Figure 1). Other- driver preference was also more frequently reported by AMD subjects than controls (37% vs. 18%, p=0.04). Presence of cataract/PCO in the better seeing eye did not show any significant association with driving cessation, restriction or other-driver preference (p>0.5 for all)
Figure 1. Driving limitations in current drivers with and without Age related macular degeneration.
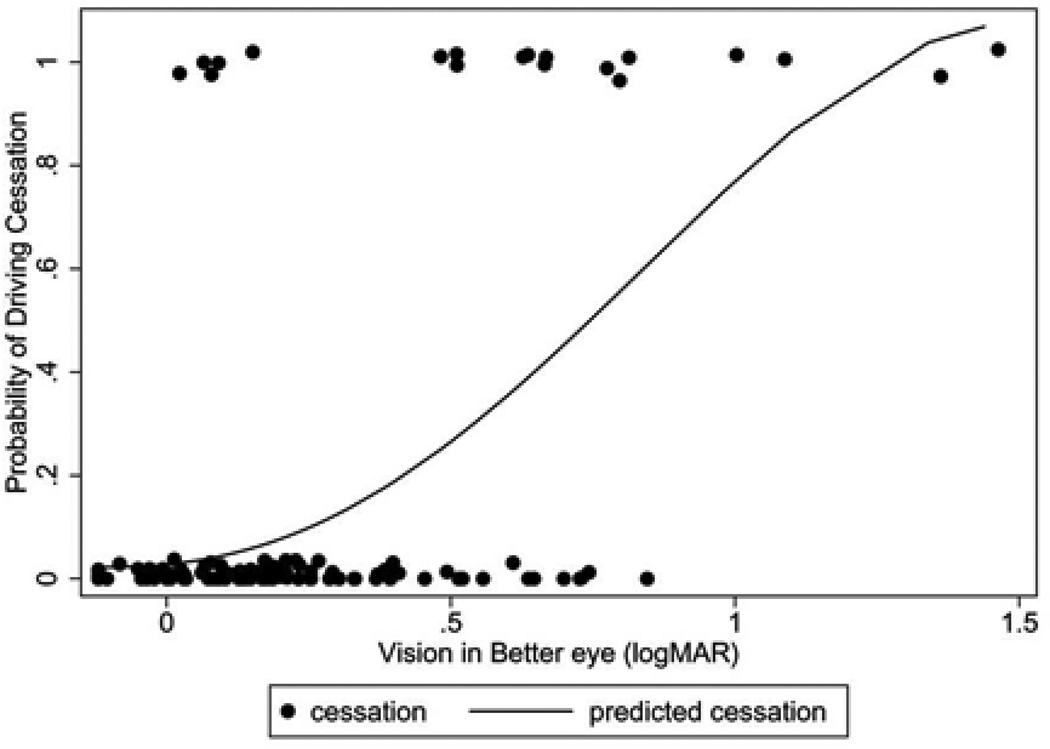
In multivariable models adjusting for age, race, gender, employment, marital status, living situation, cognition, co-morbidities, and depressive symptoms, AMD group status was not significantly associated with driving cessation (odds ratio [OR]=1.9, 95% confidence interval [CI]=0.5, 7.3, p=0.35) (Table 2). However, driving cessation was more likely with worse VA in the better-seeing eye (OR=1.5 for each 1 line loss of vision; 95% CI = 1.2, 1.9, p<0.001) and worse contrast sensitivity (OR=1.36 for each 0.1 decrement in log CS, 95% CI= 1.1, 1.7, p=0.005). Figure 2 shows the modeled probability of driving cessation based on VA amongst the full study sample. The type of AMD in the better-seeing eye (neovascular vs. non-neovascular), was not associated with driving cessation, nor were any health related or demographic factors.
Table 2.
Multivariable analysis showing effect of AMD and its severity on driving status
| Variable | Interval | Not Driving Odds Ratio (95% CI) |
Driving restriction§ Odds Ratio (95% CI) |
Pref. Another Driver§ Odds Ratio (95% CI) |
|---|---|---|---|---|
| Vision | ||||
| AMD | Present | 1.9 (0.5, 7.3) | 9.0 (2.0, 40.4)* | 2.2 (0.7, 7.1) |
| Type of AMD (worse eye) | Wet AMD | 0.6 (0.1, 3.3) | 0.4 (0.06, 1.9) | 0.3 (1.8) |
| Type of AMD (better eye) | Wet AMD | 2.7 (0.6, 11.5) | 0.7 (0.2, 2.9) | 1.2 (0.3, 4.8) |
| Binocular contrast sensitivity | 1 letter worse+ | 1.36 (1.1, 1.7)* | 2.2 (1.4, 3.5)* | 1.24 (0.9, 1.6) |
| Better eye acuity, logMAR | 1 line worse++ | 1.5 (1.2, 1.9)* | 2.5 (1.6, 4.0)* | 1.6 (1.1, 2.1)* |
| Demographics | ||||
| Age | 5 yrs older | 1.9 (0.8, 4.4) | 1.8 (0.8, 4.2) | 1.0 (0.6, 1.8) |
| Race | White | --- | 0.3 (0.03, 1.8) | 1.4 (0.3, 7.0) |
| Gender | Female | 1.1 (0.3, 4.3) | 27.9 (3.5, 224)* | 4.5 (1.2, 16.2)* |
| Employment | Present | 0.2 (0.02, 2.2) | 0.3 (0.05, 1.9) | 0.3 (0.06, 1.2) |
| Living situation | Lives with others | 0.4 (0.06, 3.1) | 3.2 (0.6, 17.1) | 2.0 (0.6, 7.2) |
| Marital Status | Married | 1.2 (0.2, 5.9) | 5.4 (0.9, 31.2) | 3.8 (1.0, 14.2)* |
| Health/cognition | ||||
| MMSE VI score | 5 points lower | 1.1 (0.2, 7.3) | 1.4 (0.2, 10.5) | 0.7 (0.1, 4.1) |
| Comorbidities | 1 illness | 1.1 (0.7, 1.6) | 0.8 (0.5, 1.3) | 1.1 (0.7, 1.6) |
| Depressive Symptoms | Present | 2.1 (0.1, 35.3) | 0.06 (0.001, 3.3) | --- |
Refers to current drivers only,
Corresponds to 0.05 log unit change,
Corresponds to 0.1 logMAR change,
P<0.05, AMD = Age related macular degeneration, Pref = Prefers, CI = Confidence interval, logMAR = logarithm of minimal angle of resolution, MMSE VI = Mini-Mental Status,
Figure 2. Modeled probability of not driving as a function of better-eye visual acuity in Age related macular degeneration patients.
In addition to better-eye visual acuity, our multivariable logistic regression model includes age, gender, unemployment, cognition, comorbidities, and depressive symptoms. logMAR = logarithm of minimum angle of resolution.
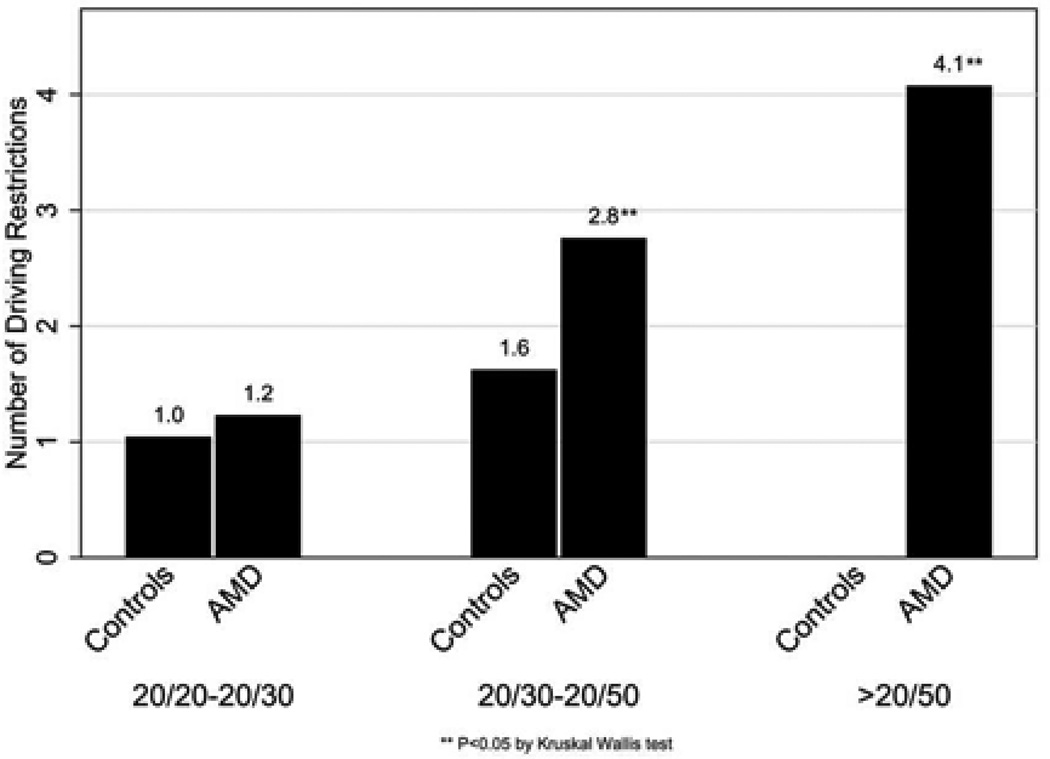
More driving restrictions were found amongst subjects with worse VA (Figure 3). Factors predictive of significant restriction (≥ 2 driving limitations) of driving included female gender (OR=27.9, 95% CI= 3.5, 223.0, p=0.002), AMD group status (OR=9.0, 95% CI= 2.0, 40.4, p=0.004), lower contrast sensitivity (OR=2.2 for each 0.1 decrement in log CS; 95%CI=1.4, 3.5, p<0.001) and worse visual acuity (OR=2.5 per 0.1 logMAR worsening, 95% CI= 1.6, 4.0, p<0.001). Other-driver preference was significantly predicted by worse vision (OR=1.6 per 0.1 logMAR increment, 95% CI= 1.2, 2.1, p=0.003), female gender (OR=4.5, 95% CI= 1.2, 16.2 p=0.02) and being married (OR=3.8, 95% CI= 1.0, 14.3, p=0.04) in multivariable models, while lower contrast sensitivity and the AMD group status did not significantly influence other-driver preference (p>0.05 for both).
Figure 3. Mean number of driving restrictions in Age related macular degeneration and control subjects.
In multivariable models including both visual acuity and contrast sensitivity, only visual acuity remained associated with driving cessation (OR=1.4, 95% CI= 1.1, 1.9, p=0.01) and driving restriction (OR=2.1, 95% CI= 1.2, 3.4, p=0.007), though these two visual measures were significantly correlated (r=0.78; p<0.001). The association between visual acuity and both driving cessation and restriction also persisted when the presence/absence of cataract/PCO was included in models.
Discussion
One in four patients with AMD-related central vision loss were noted to have stopped driving in our cohort, though patients in this group were not more likely than controls to have stopped driving in multivariable models. Driving cessation was, however, more common with worse visual acuity in the better seeing eye and contrast sensitivity, suggesting that driving cessation is more likely in more advanced disease. While most of our visually-impaired AMD patients continued to drive, they were significantly more likely to prefer that another adult drive when possible, and were more likely to restrict where, when and how far they drive. These findings suggest that driving restriction, and not driving cessation, is the primary driving adaptation found amongst patients with AMD-associated vision loss.
Our findings regarding driving cessation differ somewhat from previous papers examining driving cessation in AMD. Campbell et. al. found that about approximately 50% of their patients with AMD had ceased driving and that AMD was one of the 6 most important causes of driving cessation in the elderly. However, neither severity of AMD in terms of vision loss nor type of AMD was recorded in this study.22 DeCarlo et al. used the Driving Habits Questionnaire23 to characterize AMD patients seeking low vision rehabilitation, and reported that only 24% patients in their series continued driving.24 The lower frequency of driving cessation noted in the current study suggests that driving cessation is much more dependent on severity of vision loss in the better seeing eye rather than the mere presence/absence of AMD or AMD subtype. Indeed, many of our studied patients met the state of Maryland’s requirements for an unrestricted (at least 20/40 in one eye) or restricted (20/70 in at least one eye) driver’s license, allowing them the opportunity to continue driving if they were so inclined.
Many patients with central vision loss may adjust to driving difficulty by self regulating when, where, and how often they drive as opposed to stopping driving altogether. Of note, the apparent preference to restrict driving as a result of AMD-related vision loss, as opposed to driving altogether, is distinct from what is observed with visual field loss in glaucoma � where driving cessation is an adaptation as much or more than driving restriction.14,15Our group of visually-impaired AMD patients reported increased driving limitations, with almost one in three patients avoiding driving at night, one in two avoiding driving in unfamiliar areas and three in four avoiding driving over long distances. DeCarlo et al. found significantly reduced driving exposure and frequent avoidance of challenging driving situations amongst advanced AMD patients requiring low vision aids who continued to drive, though no control group was available for comparison.24 Ball et al, using a driving habits questionnaire, reported more driving avoidance in subjects with AMD and cataract,25 though AMD subjects comprised less than 10% (n=19) of the total sample studied. Another interview based study conducted by Moore et al. found that the older drivers with AMD used several strategies while driving, such as exercising extreme caution, relying on memory for the location of turn-offs and traffic control devices and increased scanning of the road, suggesting that driving is significantly more challenging in visually-impaired AMD patients who continue to drive.26
We found loss of visual acuity in the better seeing eye to be the best predictor for driving cessation, restriction and other driver preference. In a recent study of pooled data from the MARINA and ANCHOR trials, Bressler et al. reported that patients receiving anti-VEGF therapy for choroidal neovascularization were less likely to stop driving and had better perception of driving ability as compared to subjects receiving sham treatments. In these studies, more than 80% patients with AMD continued to drive at the 2 year follow up period, similar to our data, though the frequency of driving limitation was not explored.12 Together, these data strongly suggest that preservation of visual acuity may enable elderly subjects with AMD to sustain and enjoy their driving capabilities.
In addition to worse visual acuity in the better seeing eye, we found that reduction in contrast sensitivity was significantly associated with driving cessation and restriction but not driving preference, similar to results reported from glaucoma patients.14 Bronstad et al. in a recent study using driving simulators showed that contrast sensitivity was the best predictor for responses when the participants with AMD perceived virtual pedestrians using the areas of the visual field with no scotoma, similar to our results.27 In this study, visual acuity was not correlated with response measures i.e. late and missed responses. In our study, when both visual acuity and contrast sensitivity were included in predictive models for driving cessation and restriction, we found that only visual acuity was significantly associated with driving cessation and restriction, though both visual acuity and contrast sensitivity were highly correlated. Our current study also found that females were considerably more likely to have driving restriction and that married individuals were more likely to prefer another to drive as compared to controls. Campbell et al also showed similar trends and in their study, with females twice as likely as men to stop driving (though driving restriction and other driver preference were not measured by the authors).22 This trend suggests that women may be inherently more cautious and hence restrict themselves more than men. Also, married women probably frequently defer driving to their spouse.
One drawback of our study is the use of self reported driving habits from patients which may differ in different settings. Additionally, we used glaucoma suspects as controls in our study and not “truly” normal subjects, though previous work has demonstrated that the visual characteristics of this group are almost identical to normal subjects from population-based surveys.15 In some aspects this control group is better suited for comparison to our AMD cases since they match the AMD population with respect to seeking care at a tertiary referral care center, which may reflect very specific behavioral patterns, and have very low rates of driving cessation and driving restriction (suggesting that they are indeed visually normal despite their suspect glaucoma). Though we realize that intravitreal injections are a routine part of care for wet AMD, we excluded subjects who received intravitreal injections within one month of taking the questionnaire in order to minimize the effect of recent visual change. Given emerging evidence for the need for long-term maintenance of anti-VEGF therapy in neovascular AMD28 future studies would do well to also study patients receiving anti-VEGF therapy. Finally, since our study was cross-sectional, we cannot determine when, or at what level of visual acuity, our AMD patients stopped or restricted their driving. Strength of our study was the use of a detailed questionnaire to determine various aspects of driving. The use of this questionnaire allowed us to document the many ways that visually impaired AMD patients restrict their driving as compared to controls including distance, frequency, conditions and driver preference.
In conclusion, most of the visually-impaired AMD patients in our series continued to drive but had significant driving restrictions. Further work is necessary to evaluate whether these restrictions are effective adaptations which effectively balance safety and independence, or whether they simply indicate that patients minimize their total driving risk by driving dangerously less often.
Acknowledgments
Financial Support
This research was supported in part by the Dennis W. Jahnigen Memorial Award, NIH Grant EY018595, the Research to Prevent Blindness Robert and Helen Schaub Special Scholar Award, the Intramural Research Program of the NIH (National Institute on Aging), and the Doris Duke Charitable Research Foundation Clinical Research Fellowship. All funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicting relationship exists for any author relating to this work.
AUTHORS’ CONTRIBUTIONS
SS participated in data analysis, and drafting of the manuscript, SV participated in data collection and analysis, DF participated in study design, SSolomon, DD participated in data collection and PR participated in study design, data analysis, and oversaw conduct of the study. All authors contributed to revising the manuscript for important intellectual content, read, and approved the final manuscript.
References
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol. 1977;106:17–32. doi: 10.1093/oxfordjournals.aje.a112428. [DOI] [PubMed] [Google Scholar]
- 3.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Popescu ML, Boisjoly H, Schmaltz H, et al. Age-related eye disease and mobility limitations in older adults. Invest Ophthalmol Vis Sci. 2011;52:7168–7174. doi: 10.1167/iovs.11-7564. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. [Accessed August 11, 2013];Health Qual Life Outcomes [serial online] 2006 4:97. doi: 10.1186/1477-7525-4-97. Available at: http://www.hqlo.com/content/4/1/97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol B Psychol Sci Soc Sci. 2001;56:S343–S351. doi: 10.1093/geronb/56.6.s343. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JD, Perkins M, Ross LA, Reynolds SL. Driving status and three-year mortality among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:300–305. doi: 10.1093/gerona/gln019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman EE, Gange SJ, Munoz B, West SK. Driving status and risk of entry into long-term care in older adults. Am J Public Health. 2006;96:1254–1259. doi: 10.2105/AJPH.2005.069146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg C, Maul E, Chan ES, et al. Association of vision loss in glaucoma and age related macular degeneration with IADL disability. Invest Ophthalmol Vis Sci. 2012;53:3201–3206. doi: 10.1167/iovs.12-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finger RP, Fleckenstein M, Holz FG, Scholl HP. Quality of life in age-related macular degeneration: a review of available vision-specific psychometric tools. Qual Life Res. 2008;17:559–574. doi: 10.1007/s11136-008-9327-4. [DOI] [PubMed] [Google Scholar]
- 12.Bressler NM, Chang TS, Varma R, et al. Driving ability reported by neovascular age- related macular degeneration patients after treatment with ranibizumab. Ophthalmology. 2013;120:160–168. doi: 10.1016/j.ophtha.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Owsley C, McGwin G., Jr Driving and age-related macular degeneration. J Vis Impair Blind. 2008;102:621–635. [PMC free article] [PubMed] [Google Scholar]
- 14.van Landingham SW, Hochberg C, Massof RW, et al. Driving patterns in older adults with glaucoma. [Accessed August 11, 2013];BMC Ophthalmol [serial online] 2013 13:4. doi: 10.1186/1471-2415-13-4. Available at: http://www.biomedcentral.com/1471-2415/13/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramulu PY, West SK, Munoz B, et al. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009;116:1846–1853. doi: 10.1016/j.ophtha.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman EE, Muňnoz B, Turano KA, West SK. Measures of visual function and their association with driving modification in older adults. Invest Ophthalmol Vis Sci. 2006;47:514–520. doi: 10.1167/iovs.05-0934. [DOI] [PubMed] [Google Scholar]
- 17.Turano KA, Munoz B, Hassan SE, et al. Poor sense of direction is associated with constricted driving space in older drivers. J Gerontol B Psychol Sci Soc Sci. 2009;64:348–355. doi: 10.1093/geronb/gbp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West SK, Munoz B, Wang F, Taylor H. Measuring progression of lens opacities for longitudinal studies. Curr Eye Res. 1993;12:123–132. doi: 10.3109/02713689308999480. [DOI] [PubMed] [Google Scholar]
- 19.Findl O, Buehl W, Menapace R, et al. Comparison of 4 methods for quantifying posterior capsule opacification. J Cataract Refract Surg. 2003;29:106–111. doi: 10.1016/s0886-3350(02)01509-2. [DOI] [PubMed] [Google Scholar]
- 20.Busse A, Sonntag A, Bischkopf J, et al. Adaptation of dementia screening for vision- impaired older persons: administration of the Mini-Mental State Examination (MMSE) J Clin Epidemiol. 2002;55:909–915. doi: 10.1016/s0895-4356(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 21.Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr. 1996;8:103–112. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- 22.Campbell MK, Bush TL, Hale WE. Medical conditions associated with driving cessation in community dwelling, ambulatory elders. J Gerontol. 1993;48:S230–S234. doi: 10.1093/geronj/48.4.s230. [DOI] [PubMed] [Google Scholar]
- 23.Owsley C, Stalvey B, Wells J, Sloane ME. Older drivers and cataract: driving habits and crash risk. J Gerontol A Biol Sci Med Sci. 1999;54:M203–M211. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- 24.DeCarlo DK, Scilley K, Wells J, Owsley C. Driving habits and health-related quality of life in patients with age-related maculopathy. Optom Vis Sci. 2003;80:207–213. doi: 10.1097/00006324-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Ball K, Owsley C, Stalvey B, et al. Driving avoidance and functional impairment in older drivers. Accid Anal Prevent. 1998;30:313–322. doi: 10.1016/s0001-4575(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 26.Moore L, Miller M. Driving strategies used by older adults with macular degeneration: assessing the risks. Appl Nurs Res. 2005;18:110–116. doi: 10.1016/j.apnr.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Bronstad PM, Bowers AR, Albu A, et al. Driving with central field loss. I. Effect of central scotomas on responses to hazards. JAMA Ophthalmol. 2013;131:303–309. doi: 10.1001/jamaophthalmol.2013.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rofagha S, Bhisitkul RB, Boyer DS, et al. SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. doi: 10.1016/j.ophtha.2013.03.046. In press. [DOI] [PubMed] [Google Scholar]