Abstract
Type II diabetes and its complications are a tremendous health burden throughout the world. Our understanding of the changes that lead to glucose imbalance and insulin resistance and ultimately diabetes remain incompletely understood. Many signaling and transcriptional pathways have been identified as being important to maintain normal glucose balance, including that of the peroxisome proliferator activated receptor gamma coactivator (PGC-1) family. This family of transcriptional coactivators strongly regulates mitochondrial and metabolic biology in numerous organs. The use of genetic models of PGC-1s, including both tissue-specific overexpression and knock-out models, has helped to reveal the specific roles that these coactivators play in each tissue. This review will thus focus on the PGC-1s and recently developed genetic rodent models that have highlighted the importance of these molecules in maintaining normal glucose homeostasis.
Keywords: genetic models, PGC-1, diabetes, insulin resistance, insulin sensitivity
1. Introduction
Peroxisome proliferator activated receptor gamma coactivator alpha (PGC-1α) was first identified in a yeast two-hybrid screen looking for factors that interact with the PPARγ transcription factor in brown adipocyte cells but not in white adipocyte cells [1]. The other members of the family, PGC-1β and the more distantly related PGC-1 related coactivator (PRC), where later identified based on primary sequence homology to PGC-1α [2, 3]. These transcriptional coactivators do not bind to DNA directly, but instead are brought to DNA by interacting with a wide array of transcription factors, including most nuclear receptors[4, 5]. All three of the PGC-1s can induce a core program of mitochondrial biogenesis and oxidative phosphorylation (OXPHOS) in a variety of tissues and to varying degrees[1, 6–10, 2]. Much of this programmatic induction is achieved by binding to members of the nuclear respiratory factor (NRF) family and to the estrogen related receptor (ERR) family of transcription factors[11–13]. Binding sites for NRFs and ERRs have been identified in most of the genes involved in mitochondrial biogenesis and OXPHOS [12]. In addition to regulating the expression of nuclear-encoded genes, the PGC-1s can also induce and coordinate the expression of mitochondrial-encoded genes, at least in part by regulating the nuclear-encoded expression of the mitochondrial transcription factor A mitochondrial (Tfam) and transcription factor B (TFB) [11, 14]. In addition to the regulation of genes involved in mitochondrial biogenesis and OXPHOS the PGC-1s also regulate the expression of genes involved in fatty-acid oxidation (FAO) by interacting and coactivating members of the peroxisome proliferator activated receptor (PPAR) family of transcription factors [15]. While all three coactivators can activate similar programs of mitochondrial biogenesis and OXPHOS, numerous tissue-specific roles have also been identified (Figure 1). In the liver for example PGC-1α interacts with hepatocyte nuclear factor 4 alpha (HNF4α) and forkhead box O1 (FOXO1) to activate a gluconeogenic program while PGC-1β or PRC do not [6, 16, 17] (Figure 1). Important differences are also apparent in the variation of stimuli to which the PGC-1s respond in different tissues. For example, glucagon in the liver induces expression of PGC-1α, via activation of cAMP signaling and the CREB transcription factor[7], while having no apparent effect on PGC-1β or PRC expression.
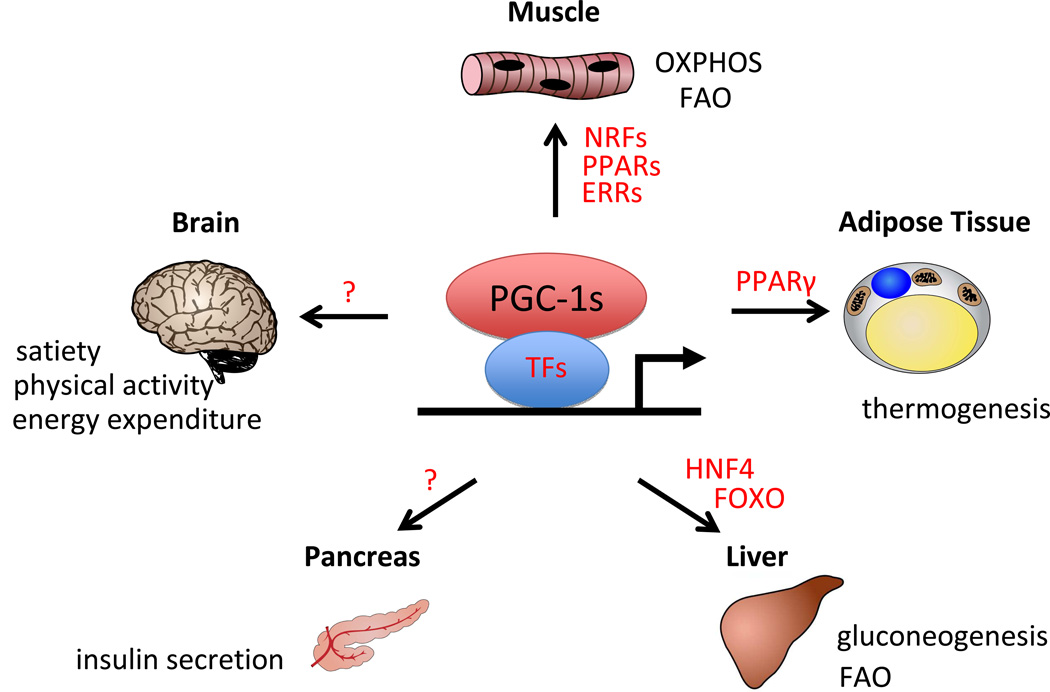
Figure 1.
Multisystem Function of PGC-1 Transcriptional Coactivators. PGC-1s interact with various transcription factors (in red) and with varying tissue specificity to activate gene signature programs to regulate glucose and energy homeostasis. See text for details.
2. PGC-1s and diabetes
Human genetic variants exist in PGC-1α that correlate with the onset of diabetes and insulin resistance[18–20], implicating PGC-1α in the development of glucose intolerance. Moreover, mitochondrial dysfunction has been repeatedly implicated in the development of insulin resistance, suggesting that defects in the PGC-1s may do the same. The observation that many PGC-1α target genes are down-regulated in human diabetes further supports this idea [21]. However, the physiological contribution of PGC-1s to the development of insulin sensitivity and glucose tolerance remains unclear, in large part because of their complex and differing roles in different tissues. Whole body deletion of either PGC-1α or PGC-1β gives rise to viable off-spring [22–25], but characterization of their metabolic phenotype has been complicated by many systemic effects (Table 1). Surprisingly, mice with whole-body deletion of PGC-1α (PGC-1α−/−) are insulin sensitive, even on a high fat diet[22]. Why this should be is not clear, and is complicated by the fact that the mice are also lean and hyperactive, both of which would be predicted also to lead to insulin sensitivity. Understanding the role of PGC-1α in glucose homeostasis therefore requires understanding its role in each relevant tissue. Work over the past few years with tissue-specific deletions of PGC-1α has begun to address this question. There is also significant functional redundancy between PGC-1α and β. Whole-body PGC-1β knockout animals (PGC-1β−/−), for example, reveal no signs of glucose intolerance or insulin sensitivity on either regular or high fat diet, despite signs of severe hepatic steatosis [23] (Table 1), which may reflect redundancy with PGC-1α. New genetic models, involving tissue-specific and both gain- and loss- of function of the PGC-1s, have now begun to paint a more clear picture of how the PGC-1s affect glucose homeostasis in each tissue.
Table 1.
Glucose homeostasis and insulin sensitivity phenotypes of PGC-1 gain- and loss-of-function genetic mouse models. See text for details.
| Tissue | Genetic Model |
PGC-1α | PGC-1β | PGC-1α/β | PRC |
|---|---|---|---|---|---|
| Whole body | Transgenic | Increased Insulin sensitivity; Glucose tolerance [75] | n/d | n/d | n/d |
| Knock-out | Insulin sensitive; lean and hyperactive [22] | No apparent phenotype [23] | n/d | Implantation Lethal [46] | |
| Hypomorphic knockout | Insulin sensitive and glucose tolerant (HFD only) [24] | No apparent phenotype [25] | Perinatal Lethal [77] | ||
| Muscle | Transgenic | Insulin resistant (HFD only) [33]; Increased Insulin sensitivity and glucose tolerant (aged and exercised animals)[34]; glucose tolerant (α4 isoform with cancer cachexia)[40] | Not reported[43] | Not reported [78] | n/d |
| Knock-out | Glucose intolerant (HFD) [41] | No apparent phenotype[44] | No apparent phenotype [44] and not reported [45] | n/d | |
| Liver | Transgenic | n/d | Not reported[57] | n/d | |
| Knock-out | Insulin resistant (heterozygote) [53] | Insulin resistant [56] | n/d | n/d | |
| Adipose | Transgenic | n/d | n/d | n/d | n/d |
| Knock-out | Insulin resistance and glucose intolerance (HFD) [61] | n/d | n/d | n/d | |
| Brain | Transgenic | Not reported[71] | n/d | n/d | n/d |
| Knock-out | No phenotype [69] | n/d | n/d | n/d | |
| Pancreas | Transgenic | Glucose intolerance [73] | n/d | n/d | n/d |
| Knock-out | n/d | n/d | n/d | n/d |
3. PGC-1s in skeletal muscle
Skeletal muscle is the primary organ for insulin-stimulated glucose clearance from the bloodstream, and is a major contributor to the development of insulin resistance and type II diabetes (T2D). In both humans and rodent models of diabetes PGC-1α expression is repressed in skeletal muscle [26, 27]. PGC-1α drives the expression of glucose transporter type 4 (GLUT4) [28] and of mitochondrial genes, which has led to the suggestion that decreased PGC-1 activity may contribute to insulin resistance in muscle by decreasing glucose transport and mitochondrial FAO, and thus causing the accumulation of incompletely oxidized fatty acid intermediates that are thought to trigger insulin resistance (Figure 1). This role for PGC-1 remains controversial, however [29, 30], and the generation of muscle-specific PGC-1 gain and loss-of-function mouse models from different groups has provided significant insight (though not simplicity) into this issue.
The most widely studied PGC-1 mouse model is the muscle-specific overexpression of PGC-1α (MCK-PGC-1αTg)[31], which uses the muscle creatine kinase promoter to drive PGC-1α. These animals exhibit increased mitochondrial biogenesis and oxidative capacity in skeletal muscle, leading to improved endurance exercise capacity [31, 32]. Initial baseline characterization of the MCK-PGC-1αTg animals revealed no differences in glucose tolerance and insulin sensitivity, despite the marked increases in mitochondrial content [31]. One possibility for the absence of phenotype was that the mice needed to be metabolically challenged in order to elicit the beneficial effects of PGC-1α. Surprisingly, however, when challenged with a high fat diet the MCK-PGC-1αTg mice proved to be insulin resistant, as assessed by hyperinsulinemic-euglycemic clamps [33](Table 1). The increased insulin resistance was likely caused by a higher content in muscle of fatty acid intermediates, in turn thought to be caused by higher PGC-1α-mediated fatty acid import into muscle. Elevation of PGC-1α in skeletal muscle, in the absence of other changes, thus surprisingly worsens glucose homeostasis. Interestingly, however, subjecting these animals to endurance exercise reverses the phenotype: the animal improve their glucose homeostasis so much so that the animals were now more insulin sensitive than wildtype controls [34]. Exercise, particularly endurance exercise, has been proven to improve glucose homeostasis [35, 36]. Thus, even though elevation of PGC-1α in skeletal muscle worsens glucose homeostasis at baseline, the same elevation strongly potentiates the beneficial effects of exercise. The mechanism for this is not clear, though it may reflect the increased capacity for fatty acid consumption once activated by an exercise stimulus. Interestingly, MCK-PGC-1αTg mice also show improved insulin sensitivity and glucose tolerance with age [37], again showing that elevation of PGC-1α in skeletal muscle is beneficial in certain contexts.
Recent work has additionally uncovered significant complexity in the transcription of the PGC-1α locus in skeletal muscle. A normally dormant alternative promoter exists 14kb upstream of the regular PGC-1α promoter. Adrenergic and other stimuli can potently activate this alternative promoter in muscle leading to transcripts containing an alternative exon1 [38, 39], and a number of alternatively spliced products [40]. Muscle specific overexpression of one of these splice products, PGC-1α4 (Myo-PGC-1α4Tg) induces muscle hypertrophy and mimics some effects of resistance exercise [40]. The Myo-PGC-1α4Tg animals at baseline have no effect on glucose homeostasis; however, when stressed with a cancer burden, these animals show improved glucose tolerance. The improvement seen in the Myo-PGC-1α4Tg animals with glucose tolerance may be due to increased insulin-like growth factor-1 (IGF-1) signaling, and decreased muscle wasting in the face of cancer (Table 1). Whether this protection would be seen in other contexts such as with high fat diet or aging is not yet known.
Analysis of skeletal muscle-specific PGC-1α knock-out (Myo-PGC-1α−/−) models has also suggested an important role for PGC-1α in maintaining normal glucose homeostasis, although genetic subtleties have complicated the interpretations of the data. Mice in which one PGC-1α allele is deleted in the whole body, while the other allele is deleted only in skeletal muscle [41], reveal a mild glucose intolerance on regular chow, which is further exacerbated when animals are placed on high fat diet. The mice were also lean and revealed increased physical activity and oxygen consumption, reminiscent of the PGC-1α total knockout animals [41]. How deletion of PGC-1α in skeletal muscle affects volition for mobility is not clear. Even more interestingly, the Myo-PGC-1α−/− animals showed signs of increased peripheral insulin sensitivity, despite the glucose intolerance, which is again divergent from the hypothesis that decreased PGC-1α in skeletal muscle contributes to insulin resistance. The glucose intolerance in these animals was in fact ascribed to inappropriately low insulin release from pancreatic islets. This observation suggests the existence of a PGC-1-mediated feedback pathway from muscle to the pancreas, but the identity of this pathway remains uncertain. More recent studies with strictly muscle-specific deletion of PGC-1α (without the total body deletion of one allele) revealed few effects on glucose homeostasis: the absence of PGC-1α blocked mitochondrial biogenesis in response to calorie restriction (CR), but had no impact on baseline glucose homeostasis, or on the marked improvements afforded by CR [42]. This observation suggests that total body deletion of one allele of PGC-1α somehow potentiates a PGC-1α-dependent cross-talk with the pancreas, although how this occurs is not known.
PGC-1β may have redundant roles with PGC-1α. The contribution of muscle PGC-1β to glucose homeostasis is less well understood, however. A muscle-specific PGC-1β mouse (MCK-PGC-1βTg) has been generated [43], but no glucose homeostasis data was reported. Analysis of a skeletal muscle-specific PGC-1β knock-out (Myo-PGC-1β−/−) animal revealed no signs of a glucose imbalance or insulin sensitivity on either normal or high fat diet [44]. Furthermore analysis of muscle-specific PGC-1β−/− in the context of a whole-body PGC-1α hypomorphic allele (Myo-PGC-1β−/−;PGC-1αKO) also did not reveal any impairment in glucose homeostasis[44] (Table 1). Conclusive evaluation of the redundant roles of PGC-1α/β in muscle homeostasis will thus require complete and muscle-specific PGC-1α/β double knock-out [45]. Finally, the role of PRC in muscle glucose homeostasis, if any, is entirely unknown. Mice lacking PRC are embryonically lethal with a placentation defect [46], and no floxed alleles have been generated.
In summary, the roles of PGC-1α and β in glucose homeostasis in the muscle remain surprisingly poorly understood, although studies in mice with muscle-specific genetic modifications are beginning to clarify the picture. PGC-1α has widely been assumed to be beneficial to insulin sensitivity in muscle, but this may be true only in the context of specific environmental stimuli, such as exercise and aging.
4. PGC-1s in the liver
Gluconeogenesis in the liver is the primary source of systemic glucose production. PGC-1α (though not PGC-1β) has been identified as being an important regulator of hepatic gluconeogenesis [17]. PGC-1α expression is relatively low during fed conditions, and increases in response to fasting and glucagon, suggesting a role in gluconeogenesis. Liver PGC-1α levels are also increased in genetic models of insulin resistance and diabetes, states of high hepatic glucose output [47, 48]. Early in vivo experiments, using primarily adenoviral delivery to the liver of PGC-1α or si-PGC-1α, showed that PGC-1α activates gluconeogenesis by inducing the expression of gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G-6Pase) [47, 6]). Induction of these genes occurs via coactivation of key transcription factors, including HNF4α and FOXO1, and can be modulated by numerous post-translational modifications, including phosphorylation by Akt [49]; and S6K [50], acetylation/deacetylation by GCN5/Sirt1 [51].
Loss of function studies in rodent models were initially somewhat puzzling, in that total body PGC-1α knockout animals had constitutively elevated hepatic gluconeogenic gene expression [22]. This likely reflected compensatory elevation of other gluconeogenic factors, including CEBPβ. As noted above, however, the phenotype of the PGC-1α KO mice is complicated by effects in numerous other tissues, thus necessitating studies with liver-specific genetic alterations in order to address liver-specific roles. More recent studies using a liver-specific PGC-1α heterozygote mouse, in which Cre recombinase was under the control of rat albumin promoter (LS-PGC-1α+/−), showed that LS-PGC-1α+/− animals, despite being able to induce PGC-1α from one allele, showed clear signs of haploinsufficiency. Hepatic fatty acid oxidation was blunted, and the livers of fasted LS-PGC-1α+/− mice showed significant increase in triglyceride and cholesterol, with a trend towards increased fatty acid accumulation that was inversely proportional to the amount of PGC-1α mRNA. The LS-PGC-1α+/− mice also showed impaired glucose release after a pyruvate tolerance test, showing that hepatic gluconeogenesis was impaired (Table 1). Interestingly, the heterozygote mice developed insulin resistance on a chow diet [52], but were protected from worse insulin resistance on a high-fat diet [53]. The latter likely occurs in part because PGC-1α, via heme synthesis and rev-erbA, inhibits hepatic fibroblast growth factor 21(FGF21), a potent circulating insulin sensitizer [53, 54]. A relatively mild but chronic decrease in hepatic PGC-1α thus blunts both gluconeogenesis and fatty acid oxidation, and has significant effects on insulin homeostasis. Interestingly, FGF21 also induces PGC-1α in a negative feedback loop, although the induction of gluconeogenic genes by FGF21 appears to be independent of PGC-1α [55]. Glucose homeostasis in these mice with homozygous liver-specific deletion of PGC-1α was not reported.
The role of PGC-1β in liver function is much less well studied. PGC-1β does not appear to coactivate the transcription factors involved in PGC-1α-driven gluconeogenesis (HNF4alpha and FOXO1) or induce the expression of gluconeogenic genes [17]. However, the generation of liver-specific PGC-1β mouse (LS-PGC-1β−/−)[56], has suggested an indirect role of PGC-1β in contributing to hepatocyte glucose homeostasis. Similar to what was observed with the LS-PGC-1α+/−, the LS-PGC-1β−/− showed increased liver triglyceride accumulation associated with a decrease in fatty-acid oxidation genes. The mitochondria from the LS-PGC-1β−/− also revealed decreased expression of OXPHOS genes and respiration capacity. Effects on the gluconeogenic response to fasting or to pyruvate tolerance test were not reported. Recently a liver-specific PGC-1β transgenic, in which PGC-1β was under the control of the apolipoprotein E promoter (LS-PGC-1βTg) has been described [57], but the effect on glucose homeostasis were also not reported (Table 1).
Taken together these models highlight the important role that PGC-1s play in maintaining normal hepatic function. PGC-1α uniquely regulates gluconeogenic genes directly, while both PGC-1s control fatty acid handling in the liver (Figure 1), with likely indirect effects on insulin sensitivity. The use of single knockouts as well as heterozygotes for the case of PGC-1α raises the question as to whether some of the mildness of the phenotypes is due to compensation by the other isoform. To truly address this question a liver-specific PGC-1 double knock-out (LS-PGC-1DKO) mouse will be required.
5. PGC-1s in Adipose Tissue
The role of adipocytes in the development of systemic insulin resistance has garnered increasing attention over the past few years, in particular as a depot of inflammatory cytokines [58]. The increase in adipose mass in obesity is associated with a decrease in mitochondrial function within the adipocytes that has also been proposed to contribute to the development of insulin resistance[59]. Patients with obesity and T2D also show decreased levels of PGC-1α mRNA in fat [60]. However, the relationship between decreased levels of PGC-1α in adipose tissue and the onset of insulin resistance is not clearly understood. PGC-1α has been well studied in brown fat, where it coactivates PPARγ to strongly induce uncoupling protein 1 (UCP1), thereby activating the thermogenic response to cold [1] (Figure 1). The role of PGC-1α in white fat has been studied less extensively. The generation of PGC-1 knockout models has recently provided increased understanding into the contribution of adipose tissue to the development of T2D and glucose imbalance.
Adipose tissue specific PGC-1α knockout (Adipo-PGC-1α−/−) were generated using an adiponectin-driven Cre recombinase [61], deleting PGC-1α in both white and brown adipose tissue. At baseline the Adipo-PGC-1α−/− are indistinguishable from control animals and exhibit no change in glucose homeostasis or insulin sensitivity. However, when challenged with a high fat diet the Adipo-PGC1α−/− animals develop excess glucose intolerance and insulin resistance, accompanied by elevated circulating lipids and cholesterol (Table 1). In hyperinsulinemic-euglycemic clamps the Adipo-PGC1α−/− exhibited decreased glucose infusion rates confirming the decreased insulin sensitivity. Interestingly, however, the Adipo-PGC1α−/− primarily had impaired insulin-stimulated hepatic glucose output, suggesting that the glucose intolerance was largely secondary to hepatic insulin resistance (rather than muscle insulin resistance). This observation suggests the existence of a PGC-1α-regulated cross-talk between fat and liver, although the molecular nature of this crosstalk is not known.
Many questions remain unanswered with regards to adipocyte-specific models of PGC-1. The use of a white and/or brown adipocyte-specific PGC-1α−/− might be informative in separating the contribution of white and brown adipose tissue to the development of the T2D. In addition, the generation of adipocyte specific overexpression of PGC-1α would also provide some insight into whether increased PGC-1α within adipocytes would increase insulin sensitivity, as might be predicted. Lastly, the contribution of PGC-1β within adipocytes in vivo is largely unknown and therefore applying similar genetic models to PGC-1β will be informative.
6. PGC-1 in the brain
The brain while not an endocrine organ in the classical sense is critical to the regulation of appetite and whole body energy homeostasis. PGC-1s have been implicated in maintaining normal brain function, and decreases in PGC-1α have been associated with Parkinson’s and Alzheimer’s [62, 63]. Epidemiological studies have also suggested that patients with Alzheimer’s have a higher incidence of diabetes [64, 65]. PGC-1α is expressed in various parts of the brain including the olfactory bulb, cerebral cortex, the substantia nigra, hippocampus and striatum [66]. Whole-body PGC-1α knockout animals are hyperactive and exhibit signs of neuronal disorders [22]. The animals are also sensitized to Parkinson disease-inducing agents [67], and have strikingly abnormal circadian rhythms [68]. These pathological effects are likely the result of large lesions in the striatum, cortex and substantia nigra of the WB-PGC-1α−/− animals [22] that may result from improper handling of reactive oxygen species.
Again, the generation of a brain-specific PGC-1α knockout (BS-PGC-1α−/−) animal, using a calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) promoter, has provided a useful tool in assessing the contribution of PGC-1α specifically in the brain to glucose homeostasis [69]. The BS-PGC-1α−/− animals develop lesions in the striatum, similar to what is observed in the total body PGC-1α. As expected, the BS-PGC-1α−/− animals maintain a normal core body temperature and have a normal thermogenic response with a cold challenge [69]. Surprisingly, however, no significant effect on locomotor activity or diurnal activity are seen in the mice, suggesting either that PGC-1α in tissues other than the brain regulates activity (reminiscent of the total-body heterozygote / muscle-specific deleted animals discussed above), or that some neurons are spared deletion of PGC-1α in the CaMKIIα transgenic animals. Equally interestingly, the BS-PGC-1α−/− animals are hypermetabolic and exhibit a significant resistance to weight gain and hepatic steatosis in response to high fat diet, despite being hyperphagic. The BS-PGC-1α−/− animals had decreased insulin levels, suggesting increased peripheral insulin sensitivity (Table 1). These studies suggest that PGC-1α in the brain regulates systemic metabolic rate (Figure 1), and can thus confer protection against high fat diet, but the mechanism remains unclear. The hypothalamus houses most of the critical neuroendocrine pathways that regulate feeding and systemic energy expenditure, including the melanocortin (MC4R) system. The fasting-induced transcriptional expression of some satiety peptides, including Agouti-related peptide (AgRP) and neuropeptide Y (NPY), was blunted in BS-PGC-1α−/− animals, implicating PGC-1α in hypothalamic function. Interestingly, in a very different context, PGC-1α and β have been demonstrated to mediate signaling in response to MC1R, the analogous system in melanocytes to MC4R in the hypothalamus [70], suggesting that the PGC-1s may play a similar role in the hypothalamus.
In summary, PGC-1α in the brain appears to be important for the regulation of feeding and systemic metabolic rate, with likely secondary effects on glucose homeostasis. PGC-1α likely acts in part in the hypothalamus, although the mechanistic specifics remain unclear. Many of these observations might also reflect the expression of CaMKIIα that was used to drive Cre expression; the use of other neuronal Cre drivers, especially targeting the hypothalamus, will be of interest. The role of PGC-1β in the brain is understudied, but whole-body deletion of PGC-1β exhibits circadian rhythms defects [23], suggesting that there may also be a role for PGC-1β in the brain. Brain specific PGC-1β−/− animals have not been reported. The generation of brain-specific PGC-1α overexpressing transgenic animals has provided a useful tool for addressing the function of PGC-1 in the brain[71], but glucose homeostasis and insulin sensitivity was not reported. Transgenic overexpression of either PGC-1β or PRC in the brain has also not been reported.
7. PGC-1 in the pancreas
The pancreas is the primary producer of insulin in the body. Increased levels of PGC-1α protein and mRNA are observed in the pancreas of obese and diabetic rodent models [72]. Increased levels of PGC-1α have also been implicated in β cell dysfunction. Adenoviral-mediated overexpression of PGC-1α in isolated rodent islets markedly suppresses glucose-stimulated insulin release, likely by reducing glucose-induced changes in ATP concentration[72]. Moreover, transplantation of PGC-1α-overexpressing islets into streptozotocin-treated animals reveal a severe hypoinsulinemia and glucose intolerance compared to transplantation of control islets [72].
The contribution of PGC-1α to islet biology was tested most recently with the generation of an inducible pancreatic PGC-1α overexpressing mouse (Ins-PGC-1αTg) in which the expression of PGC-1α is controlled by the insulin promoter [73]. This Ins-PGC-1αTg animal overexpresses PGC-1α specifically in β-cells and the expression can be regulated by administration of doxycycline, a tetracycline analog. When PGC-1α was induced during development and up to 6 months after birth, the Ins-PGC-1αTg animals showed signs of β-cell dysfunction, including decreased β-cell specific gene expression as well as decreased β-cell size. In addition, the Ins-PGC-1αTg animals showed glucose intolerance and impaired glucose-stimulated insulin release (Table 1). Interestingly, induction of PGC-1α during gestation only, i.e. switched off after birth, was sufficient to induce β-cell dysfunction in the adult Ins-PGC-1αTg animals, suggesting that PGC-1α over-expression affected β cell development in addition to the physiological response to glucose (Figure 1). In contrast, when the transgene was induced only during adulthood, the animals exhibited normal glucose tolerance as well as normal glucose-stimulated insulin release. These results suggest that the effect of PGC-1α is more pronounced during development, and that expression in the adult pancreas may have little impact on glucose homeostasis. On the other hand, the expression of β-cell specific markers was decreased in the adult-induced animals, suggesting that a longer period of PGC-1α overexpression within the adult β cells might ultimately lead to glucose imbalance.
In summary, gain-of-function studies suggest that PGC-1α suppresses pancreatic glucose-induced secretion of insulin in vivo. Loss-of-function studies would be helpful to support this conclusion, but they have not been reported. As with other organs, the potential role of PGC-1β in the pancreas has received much less attention. Knock-down of PGC-1β in a cell culture model led to an increase in glucose-stimulated insulin release[74], raising the question whether the PGC-1β would play a specific role in maintaining normal pancreatic function. β-cell specific PGC-1β models would be needed to fully address this question.
8. Conclusion
Despite more than 10 years and over 1000 articles since the discovery of PGC-1α, its role in the regulation of glucose homeostasis remains incompletely understood. The roles of PGC-1β, and especially PRC, are even less well understood. A major challenge has been to understand the different effects that the PGC-1s exert in different tissues. The recent use of tissue-specific genetic models has proven immensely useful on this point. Overall, from the standpoint of glucose tolerance, PGC-1α appears to be harmful in liver, potentially beneficial in muscle (as long as either exercising or aging), beneficial in fat, and potentially harmful in pancreas. It is therefore not at all clear what the net consequences might be of therapeutically targeting PGC-1α. Total-body PGC-1α knockout mice, which might reflect the consequences of aggressive therapeutic inhibition of PGC-1α, do not fare well, in large part due to effects on the brain[22]. Total-body PGC-1α heterozygotes, which might reflect a milder therapeutic inhibition, have not been studied extensively. Total-body overexpressing transgenic mice, on the other hand, encouragingly reveal improved glucose handling [75], although of course it can still not be said at this point precisely why. Identifying activators of PGC-1α [76] could therefore prove useful.
Acknowledgement
This work was supported by grants from the National Institutes of Health to G.C.R. (AR062128) and to Z.A. (HL094499).
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 2.Andersson U, Scarpulla RC. Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Molecular and cellular biology. 2001;21(11):3738–3749. doi: 10.1128/MCB.21.11.3738-3749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. The Journal of biological chemistry. 2002;277(3):1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- 4.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. The Journal of clinical investigation. 2006;116(3):615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 7.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 8.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. The Journal of clinical investigation. 2000;106(7):847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15(3):259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 10.Wei W, Wang X, Yang M, Smith LC, Dechow PC, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010;11(6):503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 12.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Molecular and cellular biology. 2005;25(24):10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. [Research Support, U.S. Gov't, PHS.] Molecular and cellular biology. 2005;25(4):1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Molecular and cellular biology. 2000;20(5):1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, et al. PGC- 1beta in the regulation of hepatic glucose and energy metabolism. The Journal of biological chemistry. 2003;278(33):30843–30848. doi: 10.1074/jbc.M303643200. [DOI] [PubMed] [Google Scholar]
- 18.Ek J, Andersen G, Urhammer SA, Gaede PH, Drivsholm T, Borch-Johnsen K, et al. Mutation analysis of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) and relationships of identified amino acid polymorphisms to Type II diabetes mellitus. [Research Support, Non-U.S. Gov't] Diabetologia. 2001;44(12):2220–2226. doi: 10.1007/s001250100032. [DOI] [PubMed] [Google Scholar]
- 19.Hara K, Tobe K, Okada T, Kadowaki H, Akanuma Y, Ito C, et al. A genetic variation in the PGC-1 gene could confer insulin resistance and susceptibility to Type II diabetes. [Comparative Study Research Support, Non-U.S. Gov't] Diabetologia. 2002;45(5):740–743. doi: 10.1007/s00125-002-0803-z. [DOI] [PubMed] [Google Scholar]
- 20.Oberkofler H, Linnemayr V, Weitgasser R, Klein K, Xie M, Iglseder B, et al. Complex haplotypes of the PGC-1alpha gene are associated with carbohydrate metabolism and type 2 diabetes. [Research Support, Non-U.S. Gov't] Diabetes. 2004;53(5):1385–1393. doi: 10.2337/diabetes.53.5.1385. [DOI] [PubMed] [Google Scholar]
- 21.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(12):5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3(4):e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, et al. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 2006;4(6):453–464. doi: 10.1016/j.cmet.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jove M, Salla J, Planavila A, Cabrero A, Michalik L, Wahli W, et al. Impaired expression of NADH dehydrogenase subunit 1 and PPARgamma coactivator-1 in skeletal muscle of ZDF rats: restoration by troglitazone. J Lipid Res. 2004;45(1):113–123. doi: 10.1194/jlr.M300208-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. [Controlled Clinical Trial Research Support, Non-U.S. Gov't] International journal of obesity. 2007;31(8):1302–1310. doi: 10.1038/sj.ijo.0803567. [DOI] [PubMed] [Google Scholar]
- 28.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. [Research Support, U.S. Gov't, PHS.] Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handschin C, Spiegelman BM. PGC-1 coactivators and the regulation of skeletal muscle fiber-type determination. [Comment Letter] Cell metabolism. 2011;13(4):351. doi: 10.1016/j.cmet.2011.03.008. author reply 352. [DOI] [PubMed] [Google Scholar]
- 30.Zechner C, Leone TC, Kelly DP. Response to Handschin and Spiegelman. [Response] Cell metabolism. 2011;13(4):352. [Google Scholar]
- 31.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 32.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, et al. Muscle-specific expression of PPAR{gamma} coactivator-1{alpha} improves exercise performance and increases peak oxygen uptake. Journal of applied physiology. 2008;104(5):1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 33.Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C. PGC-1alpha improves glucose homeostasis in skeletal muscle in an activity-dependent manner. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Diabetes. 2013;62(1):85–95. doi: 10.2337/db12-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce CR, Hawley JA. Improvements in insulin resistance with aerobic exercise training: a lipocentric approach. [Research Support, Non-U.S. Gov't Review] Med Sci Sports Exerc. 2004;36(7):1196–1201. [PubMed] [Google Scholar]
- 36.van Dijk JW, Manders RJ, Tummers K, Bonomi AG, Stehouwer CD, Hartgens F, et al. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. [Randomized Controlled Trial Research Support, Non-U.S. Gov't] Diabetologia. 2012;55(5):1273–1282. doi: 10.1007/s00125-011-2380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, et al. The transcriptional coactivator PGC-1{alpha} mediates exercise-induced angiogenesis in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshioka T, Inagaki K, Noguchi T, Sakai M, Ogawa W, Hosooka T, et al. Identification and characterization of an alternative promoter of the human PGC-1alpha gene. [Research Support, Non-U.S. Gov't] Biochem Biophys Res Commun. 2009;381(4):537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 40.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Cell. 2012;151(6):1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. The Journal of clinical investigation. 2007;117(11):3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finley LW, Lee J, Souza A, Desquiret-Dumas V, Bullock K, Rowe GC, et al. Skeletal muscle transcriptional coactivator PGC-1alpha mediates mitochondrial, but not metabolic, changes during calorie restriction. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):2931–2936. doi: 10.1073/pnas.1115813109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, et al. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5(1):35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12(6):633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe GC, Patten IS, Zsengeller ZK, El-Khoury R, Okutsu M, Bampoh S, et al. Disconnecting Mitochondrial Content from Respiratory Chain Capacity in PGC-1-Deficient Skeletal Muscle. Cell reports. 2013;3(5):1449–1456. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He X, Sun C, Wang F, Shan A, Guo T, Gu W, et al. Peri-implantation lethality in mice lacking the PGC-1-related coactivator protein. [Research Support, Non-U.S. Gov't] Dev Dyn. 2012;241(5):975–983. doi: 10.1002/dvdy.23769. [DOI] [PubMed] [Google Scholar]
- 47.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, PHS.] Nat Med. 2004;10(5):530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 48.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Cell Metab. 2008;7(2):125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. [Research Support, N.I.H., Extramural] Nature. 2007;447(7147):1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 50.Lustig Y, Ruas JL, Estall JL, Lo JC, Devarakonda S, Laznik D, et al. Separation of the gluconeogenic and mitochondrial functions of PGC-1{alpha} through S6 kinase. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Genes & development. 2011;25(12):1232–1244. doi: 10.1101/gad.2054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominy JE, Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Molecular cell. 2012;48(6):900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Diabetes. 2009;58(7):1499–1508. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, et al. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Endocrinology. 2011;152(8):2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chambers KT, Chen Z, Crawford PA, Fu X, Burgess SC, Lai L, et al. Liver-specific PGC-1beta deficiency leads to impaired mitochondrial function and lipogenic response to fasting-refeeding. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] PloS one. 2012;7(12):e52645. doi: 10.1371/journal.pone.0052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellafante E, Murzilli S, Salvatore L, Latorre D, Villani G, Moschetta A. Hepatic-specific activation of peroxisome proliferator-activated receptor gamma coactivator-1beta protects against steatohepatitis. [Research Support, Non-U.S. Gov't] Hepatology. 2013;57(4):1343–1356. doi: 10.1002/hep.26222. [DOI] [PubMed] [Google Scholar]
- 58.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. [Review] Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. [Research Support, N.I.H., Extramural Review] Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semple RK, Crowley VC, Sewter CP, Laudes M, Christodoulides C, Considine RV, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28(1):176–179. doi: 10.1038/sj.ijo.0802482. [DOI] [PubMed] [Google Scholar]
- 61.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Proceedings of the National Academy of Sciences of the United States of America. 2012;109(24):9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Arch Neurol. 2009;66(3):352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. [Meta-Analysis Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Sci Transl Med. 2010;2(52) doi: 10.1126/scitranslmed.3001059. 52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Podolsky S, Leopold NA, Sax DS. Increased frequency of diabetes mellitus in patients with Huntington's chorea. Lancet. 1972;1(7765):1356–1358. doi: 10.1016/s0140-6736(72)91092-6. [DOI] [PubMed] [Google Scholar]
- 65.Helisalmi S, Vepsalainen S, Hiltunen M, Koivisto AM, Salminen A, Laakso M, et al. Genetic study between SIRT1, PPARD, PGC-1alpha genes and Alzheimer's disease. [Research Support, Non-U.S. Gov't] J Neurol. 2008;255(5):668–673. doi: 10.1007/s00415-008-0774-1. [DOI] [PubMed] [Google Scholar]
- 66.Tritos NA, Mastaitis JW, Kokkotou EG, Puigserver P, Spiegelman BM, Maratos-Flier E. Characterization of the peroxisome proliferator activated receptor coactivator 1 alpha (PGC 1alpha) expression in the murine brain. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, PHS.] Brain Res. 2003;961(2):255–260. doi: 10.1016/s0006-8993(02)03961-6. [DOI] [PubMed] [Google Scholar]
- 67.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC- 1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 69.Ma D, Li S, Lucas EK, Cowell RM, Lin JD. Neuronal inactivation of peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) protects mice from diet-induced obesity and leads to degenerative lesions. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] The Journal of biological chemistry. 2010;285(50):39087–39095. doi: 10.1074/jbc.M110.151688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A, et al. PGC-1 coactivators regulate MITF and the tanning response. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Molecular cell. 2013;49(1):145–157. doi: 10.1016/j.molcel.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mudo G, Makela J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, et al. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. [Research Support, Non-U.S. Gov't] Cell Mol Life Sci. 2012;69(7):1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, et al. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, PHS.] Developmental cell. 2003;5(1):73–83. doi: 10.1016/s1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 73.Valtat B, Riveline JP, Zhang P, Singh-Estivalet A, Armanet M, Venteclef N, et al. Fetal PGC-1alpha overexpression programs adult pancreatic beta-cell dysfunction. [Research Support, Non-U.S. Gov't] Diabetes. 2013;62(4):1206–1216. doi: 10.2337/db12-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oberkofler H, Hafner M, Felder T, Krempler F, Patsch W. Transcriptional co-activator peroxisome proliferator-activated receptor (PPAR)gamma co-activator-1beta is involved in the regulation of glucose-stimulated insulin secretion in INS-1E cells. [Research Support, Non-U.S. Gov't] Journal of molecular medicine. 2009;87(3):299–306. doi: 10.1007/s00109-008-0425-0. [DOI] [PubMed] [Google Scholar]
- 75.Liang H, Balas B, Tantiwong P, Dube J, Goodpaster BH, O'Doherty RM, et al. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab. 2009;296(4):E945–E954. doi: 10.1152/ajpendo.90292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arany Z, Wagner BK, Ma Y, Chinsomboon J, Laznik D, Spiegelman BM. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1alpha and oxidative phosphorylation. [Research Support, N.I.H., Extramural] Proceedings of the National Academy of Sciences of the United States of America. 2008;105(12):4721–4726. doi: 10.1073/pnas.0800979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, et al. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes & development. 2008;22(14):1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. [Research Support, Non-U.S. Gov't] PloS one. 2011;6(12):e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]