Abstract
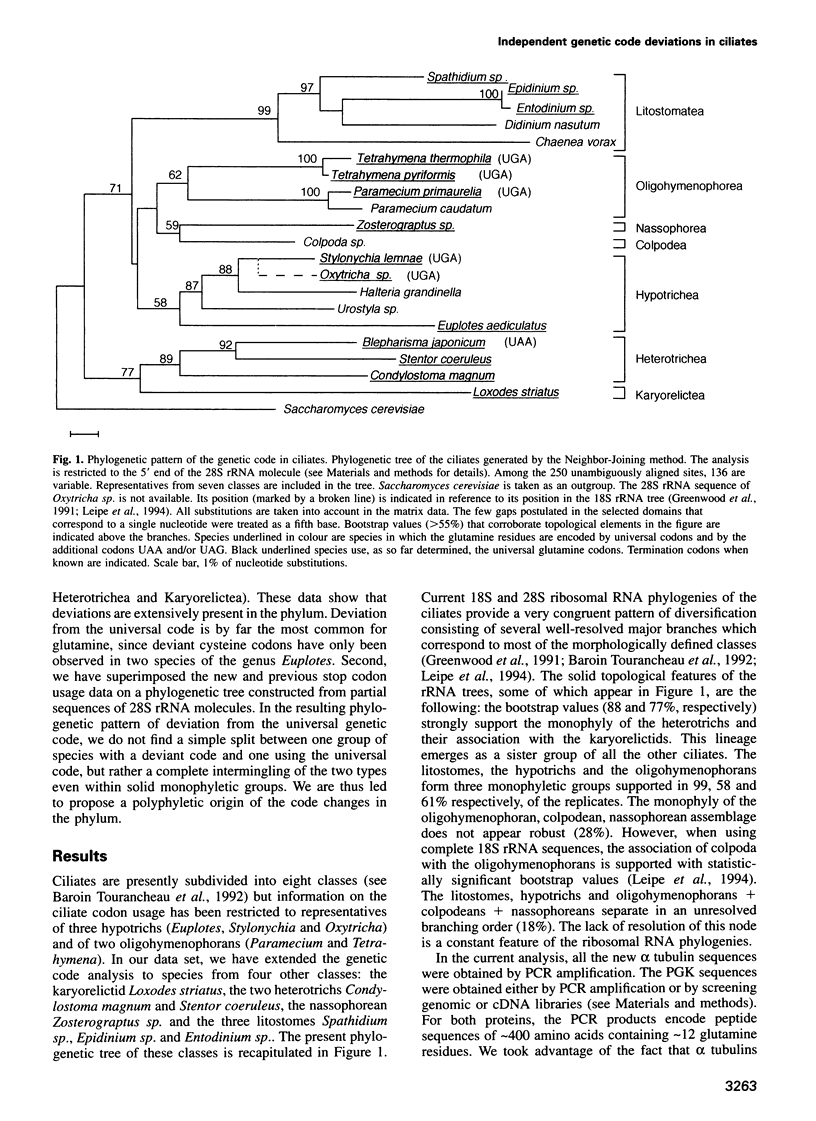
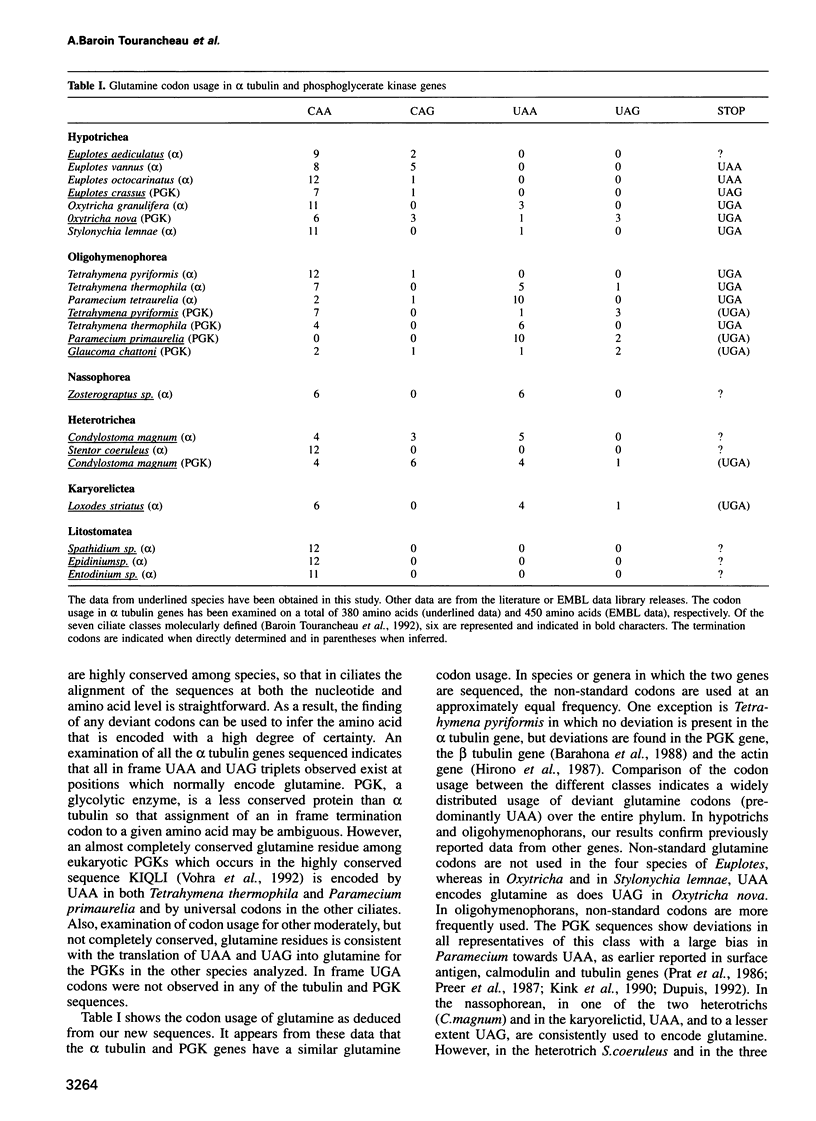
In several species of ciliates, the universal stop codons UAA and UAG are translated into glutamine, while in the euplotids, the glutamine codon usage is normal, but UGA appears to be translated as cysteine. Because the emerging position of this monophyletic group in the eukaryotic lineage is relatively late, this deviant genetic code represents a derived state of the universal code. The question is therefore raised as to how these changes arose within the evolutionary pathways of the phylum. Here, we have investigated the presence of stop codons in alpha tubulin and/or phosphoglycerate kinase gene coding sequences from diverse species of ciliates scattered over the phylogenetic tree constructed from 28S rRNA sequences. In our data set, when deviations occur they correspond to in frame UAA and UAG coding for glutamine. By combining these new data with those previously reported, we show that (i) utilization of UAA and UAG codons occurs to different extents between, but also within, the different classes of ciliates and (ii) the resulting phylogenetic pattern of deviations from the universal code cannot be accounted for by a scenario involving a single transition to the unusual code. Thus, contrary to expectations, deviations from the universal genetic code have arisen independently several times within the phylum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S. G., Kurland C. G. Codon preferences in free-living microorganisms. Microbiol Rev. 1990 Jun;54(2):198–210. doi: 10.1128/mr.54.2.198-210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird S. E., Klobutcher L. A. Genetic characterization and use of a restriction fragment length variant in the hypotrichous ciliate Euplotes crassus. J Protozool. 1988 Nov;35(4):459–465. doi: 10.1111/j.1550-7408.1988.tb04130.x. [DOI] [PubMed] [Google Scholar]
- Barahona I., Soares H., Cyrne L., Penque D., Denoulet P., Rodrigues-Pousada C. Sequence of one alpha- and two beta-tubulin genes of Tetrahymena pyriformis. Structural and functional relationships with other eukaryotic tubulin genes. J Mol Biol. 1988 Aug 5;202(3):365–382. doi: 10.1016/0022-2836(88)90271-9. [DOI] [PubMed] [Google Scholar]
- Baroin-Tourancheau A., Delgado P., Perasso R., Adoutte A. A broad molecular phylogeny of ciliates: identification of major evolutionary trends and radiations within the phylum. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9764–9768. doi: 10.1073/pnas.89.20.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroin A., Perasso R., Qu L. H., Brugerolle G., Bachellerie J. P., Adoutte A. Partial phylogeny of the unicellular eukaryotes based on rapid sequencing of a portion of 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1988 May;85(10):3474–3478. doi: 10.1073/pnas.85.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F. Eucaryotic codes. Experientia. 1990 Dec 1;46(11-12):1106–1117. doi: 10.1007/BF01936920. [DOI] [PubMed] [Google Scholar]
- Doak T. G., Doerder F. P., Jahn C. L., Herrick G. A proposed superfamily of transposase genes: transposon-like elements in ciliated protozoa and a common "D35E" motif. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):942–946. doi: 10.1073/pnas.91.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood S. J., Schlegel M., Sogin M. L., Lynn D. H. Phylogenetic relationships of Blepharisma americanum and Colpoda inflata within the phylum ciliophora inferred from complete small subunit rRNA gene sequences. J Protozool. 1991 Jan-Feb;38(1):1–6. doi: 10.1111/j.1550-7408.1991.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Hanyu N., Kuchino Y., Nishimura S., Beier H. Dramatic events in ciliate evolution: alteration of UAA and UAG termination codons to glutamine codons due to anticodon mutations in two Tetrahymena tRNAs. EMBO J. 1986 Jun;5(6):1307–1311. doi: 10.1002/j.1460-2075.1986.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hirono M., Endoh H., Okada N., Numata O., Watanabe Y. Tetrahymena actin. Cloning and sequencing of the Tetrahymena actin gene and identification of its gene product. J Mol Biol. 1987 Mar 20;194(2):181–192. doi: 10.1016/0022-2836(87)90367-6. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Doktor S. Z., Frels J. S., Jaraczewski J. W., Krikau M. F. Structures of the Euplotes crassus Tec1 and Tec2 elements: identification of putative transposase coding regions. Gene. 1993 Oct 29;133(1):71–78. doi: 10.1016/0378-1119(93)90226-s. [DOI] [PubMed] [Google Scholar]
- Jukes T. H., Bessho Y., Ohama T., Osawa S. Release factors and the genetic code. Nature. 1991 Aug 15;352(6336):575–575. doi: 10.1038/352575a0. [DOI] [PubMed] [Google Scholar]
- Jukes T. H., Osawa S., Muto A. Divergence and directional mutation pressures. Nature. 1987 Feb 19;325(6106):668–668. doi: 10.1038/325668b0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Honda H., Taniguchi-Morimura J., Iwasaki S. The codon CUG is read as serine in an asporogenic yeast Candida cylindracea. Nature. 1989 Sep 14;341(6238):164–166. doi: 10.1038/341164a0. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Maley M. E., Preston R. R., Ling K. Y., Wallen-Friedman M. A., Saimi Y., Kung C. Mutations in paramecium calmodulin indicate functional differences between the C-terminal and N-terminal lobes in vivo. Cell. 1990 Jul 13;62(1):165–174. doi: 10.1016/0092-8674(90)90250-i. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Liang A., Heckmann K. Blepharisma uses UAA as a termination codon. Naturwissenschaften. 1993 May;80(5):225–226. doi: 10.1007/BF01175738. [DOI] [PubMed] [Google Scholar]
- Meyer F., Schmidt H. J., Heckmann K. Pheromone 4 gene of Euplotes octocarinatus. Dev Genet. 1992;13(1):16–25. doi: 10.1002/dvg.1020130104. [DOI] [PubMed] [Google Scholar]
- Meyer F., Schmidt H. J., Plümper E., Hasilik A., Mersmann G., Meyer H. E., Engström A., Heckmann K. UGA is translated as cysteine in pheromone 3 of Euplotes octocarinatus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3758–3761. doi: 10.1073/pnas.88.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Jukes T. H. Codon reassignment (codon capture) in evolution. J Mol Evol. 1989 Apr;28(4):271–278. doi: 10.1007/BF02103422. [DOI] [PubMed] [Google Scholar]
- Osawa S., Jukes T. H., Watanabe K., Muto A. Recent evidence for evolution of the genetic code. Microbiol Rev. 1992 Mar;56(1):229–264. doi: 10.1128/mr.56.1.229-264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe H. MUST, a computer package of Management Utilities for Sequences and Trees. Nucleic Acids Res. 1993 Nov 11;21(22):5264–5272. doi: 10.1093/nar/21.22.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A., Katinka M., Caron F., Meyer E. Nucleotide sequence of the Paramecium primaurelia G surface protein. A huge protein with a highly periodic structure. J Mol Biol. 1986 May 5;189(1):47–60. doi: 10.1016/0022-2836(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Rudman B., Barnett A. Molecular biology of the genes for immobilization antigens in Paramecium. J Protozool. 1987 Nov;34(4):418–423. doi: 10.1111/j.1550-7408.1987.tb03205.x. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The DNA of ciliated protozoa. Microbiol Rev. 1994 Jun;58(2):233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schneider S. U., Leible M. B., Yang X. P. Strong homology between the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase of two species of Acetabularia and the occurrence of unusual codon usage. Mol Gen Genet. 1989 Sep;218(3):445–452. doi: 10.1007/BF00332408. [DOI] [PubMed] [Google Scholar]
- Schultz D. W., Yarus M. Transfer RNA mutation and the malleability of the genetic code. J Mol Biol. 1994 Feb 4;235(5):1377–1380. doi: 10.1006/jmbi.1994.1094. [DOI] [PubMed] [Google Scholar]
- Vohra G. B., Golding G. B., Tsao N., Pearlman R. E. A phylogenetic analysis based on the gene encoding phosphoglycerate kinase. J Mol Evol. 1992 May;34(5):383–395. doi: 10.1007/BF00162995. [DOI] [PubMed] [Google Scholar]


