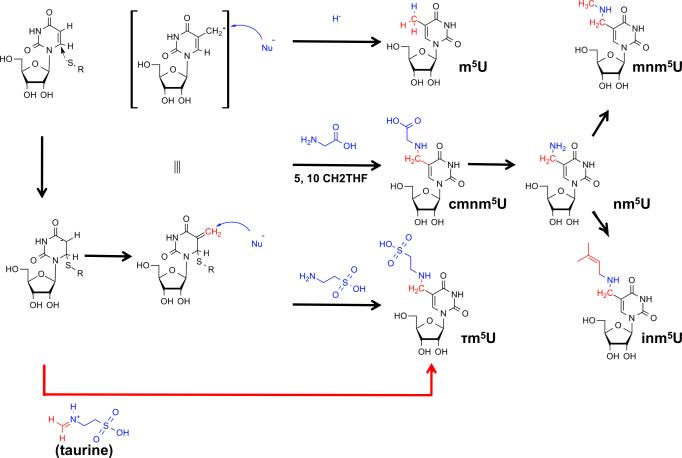
Figure 3. Chemical Strategies for C5 Modification of Uridines in Bacteria.
The C5 position of uridines is rendered nucleophilic by Michael addition of a cysteine thiolate to the 5,6 double bond. 5,10-methylene tetrahydrofolate (5,10-CH2THF) provides a C1-body at the oxidation state of formaldehyde which acts as electrophile (red), leading to an electrophilic 5-methyleneuridine intermediate. This intermediate can be attacked by a variety of nucleophiles (blue), which may include hydride (from THF), or different aminoacids such as glycine (bacteria) or taurine (metazoan mitochondria). After dealkylation of cmnm5U, the resulting 5-aminomethyluridine (nm5U) can be methylated to mnm5U or prenylated to inm5U. The latter reaction is unconfirmed.