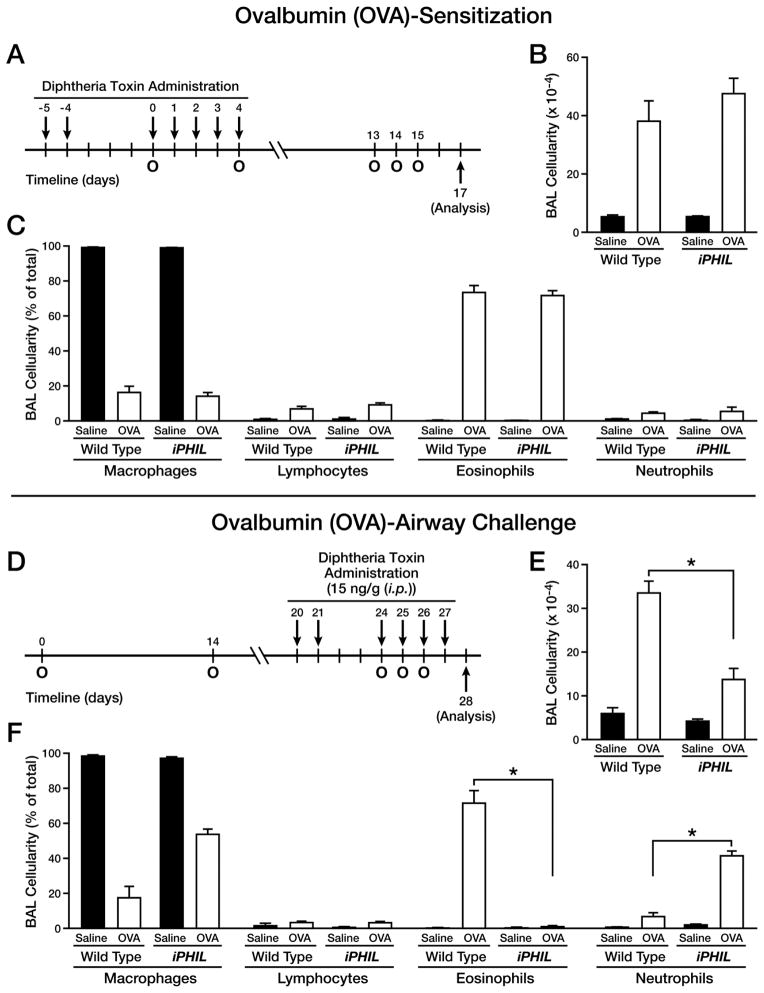
Figure 3. Eosinophil ablation during OVA challenge, but not the sensitization, phase resulted in a neutrophilic airway phenotype.
Wild type and iPHIL mice were administered DT ((i.p.) - 15ng/gram body weight) prior to and during the sensitization and airway challenge phases of an acute OVA allergen provocation protocol to assess the consequences of eosinophil ablation. (A) Eosinophil ablation during OVA sensitization: DT was administered (i.p.) on days -5, -4, and 0–4 (arrows) with day 0 representing the first of two (day 0 and 4) OVA sensitizations (O). These mice were then challenged with an OVA nebulant generated from a 1% OVA solution in saline (control mice received saline vehicle alone) on protocol days 13–15 (O) and assessed on day 17. (B) Total BAL cell counts as well as (C) cell differential analyses were performed on cytospins preparations of recovered airway cells (mean ± SEM), counting >300 cells/sample. *P<0.05. (D) Eosinophil ablation during OVA airway challenge: DT was administered on days 20, 21, and 24–27 (arrows) with day 0 representing the first of two (day 0 and 14) OVA sensitizations (intraperitoneal (i.p.) administration of OVA/Imject® adjuvant (o)) and days 24–26 representing the airway OVA challenge phase of this acute protocol (OVA nebulant generated from a 1% OVA solution in saline (o)) with mice assessed on day 28; control mice received saline vehicle alone. (E) Total BAL cell counts as well as (F) cell differential analyses were performed on cytospins preparations of recovered airway cells (mean ± SEM), counting >300 cells/sample. *P<0.05.