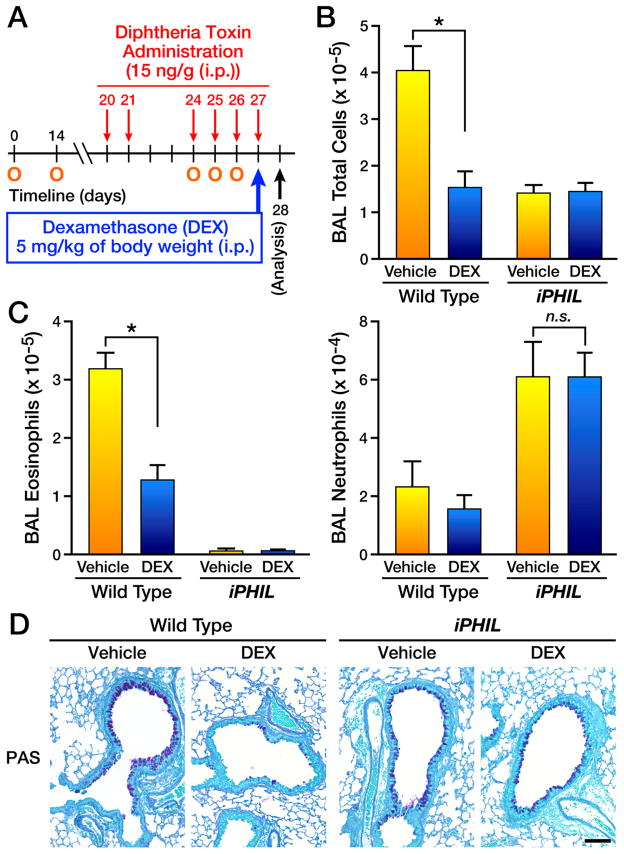
Figure 6. Eosinophil ablation during allergen challenge leads to a steroid-resistant neutrophilic phenotype.
(A) Wild type and iPHIL mice were sensitized (i.p.) with OVA/Imject® adjuvant protocol days 0 and 14 (O) and administered DT ((i.p.) - 15ng/gram body weight) prior to and during the airway challenge phases on days 20, 21, and 24–27 (arrows) with the mice receiving an OVA nebulant generated from a 1% OVA solution in saline (control mice received saline alone) on protocol days 24–26 (O) and assessed on day 28. Some mice received saline (Vehicle) while other mice received (i.p.) 5mg/kg body weight dexamethasone (DEX) on day 27 of the protocol. (B) Total bronchoalveolar lavage (BAL) cellularity in wild type vs. DT-treated iPHIL mice following treatment with DEX (control animals received saline vehicle alone (Vehicle)). *p<0.05 (C) Cell counts and differentials were used to determine the number (mean ± SEM) of airway eosinophils and neutrophils following treatment with DEX (control animals received saline vehicle alone (Vehicle)). *p<0.05; n.s., not significant. (D) Representative photomicrographs of lung sections are presented from wild type as compared to iPHIL mice whose eosinophils were ablated during the airway challenge phase. Lung sections were stained with PAS and showed that although wild type allergen-treated lungs displayed a reduction in goblet cell metaplasia/airway epithelial cell mucin accumulation ((PAS) – black staining cells) upon DEX treatment, allergen-treated iPHIL mice receiving dexamethasone displayed inflammation levels similar to saline injected animals; control animals received saline vehicle alone. Scale bar = 100μm.