Abstract
The vitamin D endocrine system has clear beneficial effects on bone as demonstrated by prevention of rickets in children and by reducing the risk of osteomalacia or osteoporosis in adults or elderly subjects. Depending on the design of the study of genetically modified animals, however, 1,25(OH)2D and the vitamin D receptor (VDR) may have no effect, beneficial or even deleterious direct effects on bone. We present here a comprehensive model of the direct effects of vitamin D on bone. In case of sufficient calcium supply, vitamin D and its metabolites can improve the calcium balance and facilitate mineral deposition in bone matrix largely without direct effects on bone cells, although some beneficial effects may occur via mature osteoblasts, as demonstrated in mice with osteoblast-specific overexpression of VDR or 1α-hydroxylase. In case of calcium deficiency, however, 1,25(OH)2D enhances bone resorption, whereas simultaneously inhibiting bone mineralization, so as to defend serum calcium homeostasis at the expense of bone mass. This dual role probably provides a survival benefit for land vertebrates living in a calcium-poor environment.
Introduction
Vitamin D has a well-recognized role in bone biology, being required for normal bone formation and normal mineralization. The uncertainty that will be addressed in this review is how much of its effects on bone are secondary to its actions on gut calcium and phosphate absorption and how much relate to direct effects on bone. Moreover, if there are effects directly on bone, how much of any bone activity is on bone formation and how much on bone resorption. Conflicting data suggest that these actions may differ by timing, skeletal site and dietary calcium intake.
In in vivo studies, in vitamin D receptor knock out (Vdr−/−) models, there was the expected phenotype similar to various forms of vitamin D-deficient or -resistant rickets. There were similar phenotypes in models of knockout of the 1α-hydroxylase (CYP27B1) enzyme. The findings in these studies underpin the critical role of vitamin D in normal calcium and bone/tooth/growth plate homeostasis. Vitamin D is generally associated not only with improved bone mineralization but also with increased bone resorption, and thus may seem to represent ‘good' and ‘bad' effects on bone. In vitro studies have readily demonstrated bone resorbing effects responses to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), as shown by elegant studies in Suda's laboratory,1 whereas it has been more difficult to demonstrate unequivocal beneficial effects of vitamin D metabolites on bone formation (see this issue, van Driel and van Leeuwen2).
In this review, we try to define the direct effects of the vitamin D endocrine system on bone homeostasis based on results generated in transgenic animal models. It is important to be aware that the knockout models that are osteoblast specific have generally used the collagen Iα1 2.3 kb promoter that is expressed very widely in cells of the osteoblast lineage as well as chondrocytes.3 This contrasts with the osteocalcin promoter that is more specifically targeted to mature cells of the osteoblast lineage, including osteocytes and hypertrophic chondrocytes.4 The specificity of expression of the osteocalcin4 and commonly used collagen Iα1 promoter fragments is not as clear-cut as has been assumed. This ‘infidelity' of expression may explain some of the divergent findings in models that seem otherwise similar, if not identical. Depending on the model, three different conclusions can be drawn: vitamin D has no, has a beneficial or has a deleterious effect on bone. We will first review the different arguments and then present a model to explain these apparently conflicting observations.
Possible scenarios for vitamin D's direct action on bone
Scenario 1: the vitamin D hormone has indirect but no direct effects on bone
Mice with global VDR deficiency raised on a high calcium or rescue (high calcium and lactose) diet were found to have a normal calcium homeostasis, normal bone and growth plate morphology and normal bone resorption/formation. Indeed, dissecting the role of the VDR in the rickets-osteomalacia phenotype in Vdr−/− mice, a high calcium-phosphate-lactose diet prevented any clear bone phenotype5,6,7,8,9,10,11,12,13 (Table 1). This clearly points towards an indirect effect of vitamin D on bone by facilitating the intestinal absorption of calcium. This is confirmed by the restoration of normal bone structure in animals with global Vdr−/− PLUS selective reintroduction of VDR in the intestine.14,15,16,17 Similar conclusions can be drawn from animals with global Cyp27b1−/− raised on a rescue diet.18,19,20 Indeed, Cyp27b1−/− mice fed a rescue diet maintained a normal serum calcium concentration and relatively normal bone structure and histology despite undetectable serum 1,25(OH)2D3 levels.21 By contrast, in another VDR−/− model, even with the high calcium rescue diet, there were persisting skeletal abnormalities with wider growth plates, shorter bones and lesser mineral apposition rate.22
Table 1. Synopsis of bone effects observed in transgenic VDR mouse models.
| Reference | Model | Age at testing | Cortical bone | Trabecular bone | Mechanisms |
|---|---|---|---|---|---|
| Amling et al.8 | Vdr−/− (no rescue diet) | 70 days | >85% bone surface ↑↑ BV/TV | ||
| Vdr−/− (rescue diet) | 70 days | No Δ | No Δ | ||
| Gardiner et al.26 | OS-VDR | 4 and 9 months | 5% wider (tibia) | 20% ↑ in BV/TV (caudal vert) | 30% ↓ in resorption surface |
| Panda et al.20 | Cyp27b1−/− & Vdr−/− (rescue diet) | 4 months | ∼50% ↓ in BV/TV (tibia) | ↓ in mineral apposition rate | |
| Masayuma et al.35 | Chondrocyte Vdr−/− | 15 days 8 weeks | 50% ↓ in BV/TV No Δ | ↑ in FGF-23 & 1,25(OH)2D3 | |
| Xue et al.14 | Intestinal VDR rescue of Vdr−/− | 18 weeks | 18% ↑ | 18% ↑ | ↑ Intestinal calcium absoprtion? |
| Lieben et al.15 | Osteocyte Vdr−/− | 14 weeks | No Δ | No Δ | |
| Intestinal Vdr−/− | 14 weeks | 60% ↓ | ↓↓ | ↑ Resorption ↓ mineralization | |
| Yamamoto et al.34 | Vdr−/− (Rescue diet) | 18 weeks | No Δ | ||
| Heterozygote Vdr−/+ (rescue diet) | 18 weeks | 20% ↑ in BV/TV (tibia) | |||
| Osteoblast Vdr−/− | 18 weeks | No Δ | 30% ↓ in resorption surface |
Abbreviations: ↑, increase; ↓, decrease; Δ, difference; BV, bone volume; TV, trabecular volume; VDR, vitamin D receptor.
Using a different approach again a similar conclusion of absence of direct effects on bone is reached by analysis of mice with osteocyte (and late osteoblast)-specific Vdr−/−.15 These mice have relatively normal bone histology and mass and normal gene expression patterns in bone, unlike global Vdr−/− mice (Table 1). The overall interpretation from these data is that bone mineral deposition/formation and bone resorption can be relatively normal as long as serum calcium and phosphate and calciotropic hormone levels are normal. This would suggest that the vitamin D endocrine system does not have an essential role in bone resorption although there is disagreement, depending upon the model used, whether it has an essential role in bone formation.
Scenario 2: the vitamin D hormone is good for bone
Numerous observations in humans and in fact in nearly all terrestrial vertebrates (from amphibians, reptiles, birds up to mammals) have clearly demonstrated that vitamin D is essential to prevent rickets in growing animals (see Bouillon and Suda23 published in first issue (January 2014) of BoneKEy Reports), and most studies also demonstrated its essential role to prevent osteomalacia in adult humans or animals (reviewed in Pettifor and Prentice24). In fact, the very first proof of the existence of vitamin D was made in rachitic dogs.25 The most obvious effect of vitamin D in these models was enhanced mineral deposition in osteoid tissues. However, these models cannot distinguish direct from indirect effects. In studies of overexpression of the VDR in mature cells of the osteoblastic lineage, there was an increase in both cortical and trabecular bone.26 The overexpression model of VDR in osteoblasts used an osteocalcin-based promoter that was expressed in late-stage osteoblasts as well as osteocytes and hypertrophic chondrocytes.4 Interestingly, in osteoblast overexpression of the VDR there was both an increase in formation and a decrease in resorption (Table 1). In studies exploring possible mechanisms of the effect of overexpression of VDR in mature osteoblastic cells, these mature osteoblastic cells also had lesser ability to activate functional osteoclasts in vitro.27 The difference in outcomes by changing VDR expression in different cell types of the osteoblastic lineage is comparable to differences in outcomes from overexpression of Notch driven by the 3.6 kb fragment or the 2.3 kb fragment of the collagen Iα1 promoter. In these models, overexpression of Notch in early osteoblast lineage cells led to osteopenia,28,29,30 whereas overexpression in osteocytes led to formation of abundant woven bone. The latter formation of woven bone was associated with relatively low osteoclast numbers.30 Studying the osteocalcin-driven osteoblast-specific VDR overexpression model in vitro, there was evidence of suppression of activation of osteoclasts in a co-culture model.27 This effect could relate to overall changes in gene expression patterns, such as osteoprotegerin, but these were not examined. However, irrespective of the mechanism this in vitro finding fitted with the in vivo findings, as noted above, of increased bone mass with decreased resorption surface.26 These findings are also consistent with the findings in vivo of fewer osteoblasts and lesser mineral apposition rate in Vdr−/− (and Cyp27b1−/−) mice22 and in bone marrow cultures in vitro from Vdr−/− (and Cyp27b1−/−) mice, in which there were fewer total and fewer mineralized colonies formed.22 However, in another similar study Vdr−/− calvarial osteoblasts produced more mineralizing colonies consistent with accelerated osteoblast differentiation.5,31 One difference between these models is the developmental stage of the osteoblast lineage at which the knockout or transgene was expressed. In mice with osteoblast-specific overexpression of CYP27B1, bone mass in the lumbar spine and femoral metaphysis was also increased by 10% at the age of 20 weeks mainly by increased bone formation.32
Overall, these data suggest that VDR, at least during the later stages of osteoblast development, has positive direct effects on bone as both formation is increased and resorption downregulated.
Scenario 3: the active vitamin D hormone has or can have negative direct effects on bone
The absence of VDR in osteoblasts has been shown to result in increased bone mass. This was first observed when bone samples from normal or Vdr−/− mice were transplanted in normal or Vdr−/− mice.31,33 Normal bone transplanted into Vdr−/− mice showed decreased mineralization, whereas bone mineral content was increased in Vdr−/− bone tissues transplanted in wild-type mice.31,33 Yamamoto et al.34 noted that heterozygote ablation of the VDR had a phenotype of increased bone mass. In analyses of the apparent mechanism, they observed a downregulation of osteoclast activity rather than an increase in bone formation. The increase in bone mass in global heterozygous Vdr−/+ mice was modest. Interestingly, the effect was greater in osteoblast-specific Vdr−/− mice (using a 2.3 kb collagen Iα1 promoter). This reduced the VDR gene in all cells of the osteoblastic lineage as well as chondrocytes.34 Further analysis indicated that VDR in osteoblasts could act as a negative regulator of bone mass through stimulation of RANKL (receptor activator of nuclear factor-κB ligand)-induced osteoclastogenesis. These negative effects on bone may be transient as in both the Cyp27b1−/− and Vdr−/− models raised on a rescue diet but followed into older age for mice, there was evidence of reversal of the higher bone mass phenotype;22 in fact, trabecular bone volume fell below wild-type bone volume.
Another model that bears on these mechanisms is that of the Vdr−/− in chondrocytes, using a Cre-recombinase driven by collagen IIα1.35 In that model, there was a transient increase in bone volume in neonates and at 15 days. However, this was no longer apparent by 8 weeks of age, when the growth plate had largely lost its activity. These transient changes were possibly due to decreased osteoclast numbers and activity, which probably also related to delayed vascular invasion with decreased vascular endothelial growth factor expression.35 Moreover, these changes were associated with transient increases in both fibroblast growth factor-23 and 1,25(OH)2D3 levels, due to an unknown humoral factor transmitting the VDR message from chondrocytes to bone cells and the kidney. Remarkably, similar results were obtained in mice with selective chondrocyte-specific deletion of Cyp27B1 (using a collagen IIα1 promotor). This deletion that prevented local production of 1,25(OH)2D3 decreased the expression of VGEF, delayed vascularization, decreased RANKL expression, decreased osteoclastogenesis and increased trabecular bone mass.36,37 These data support a role of VDR and local production of 1,25(OH)2D3 in chondrocytes and endochondrial ossification, with overall negative effects on bone mass as long as the growth plate remains active.38,39 On the other hand, the vitamin D response element was first identified in the osteocalcin gene,40,41 and 1,25(OH)2D3 activated the gene. In the context of osteocalcin as one of the primary markers of osteoblast activity and bone formation, this is also consistent with 1,25(OH)2D3 having a positive effect on bone formation. Similarly, the reported upregulation of the Wnt co-receptor LRP-5 also supports the potential of a positive effect of 1,25(OH)2D3 on bone formation.42
Selective deletion of VDR in intestine (using a villin promotor Cre system) caused calcium malabsorption similar to that of global Vdr−/− mice, but the bone phenotype was substantially worse in intestine-selective Vdr−/− mice, indicating that the presence of VDR in bone cells allowed a physiological increase in bone resorption and decrease in bone mineral deposition in the presence of high parathyroid hormone (PTH) and 1,25(OH)2D3 serum concentrations.15 Presumably, due to persistence of the physiological bone resorption response to both PTH and 1,25(OH)2D3, intestine-selective Vdr−/− mice were able to maintain normal serum calcium and phosphate concentrations, whereas total knockout mice displayed the expected low calcium and phosphate levels. These studies demonstrate the critical and major role of vitamin D (in conjunction with PTH) in overall calcium and phosphate homeostasis rather than purely bone homoeostasis.
The mechanisms whereby 1,25(OH)2D3 through the VDR increases bone resorption are well understood.1 There is also evidence that this pathway may also inhibit bone mineral deposition as shown in vivo15 and in vitro.43,44,45 The molecular mechanisms involve several genes regulating (positively) the production or (negatively) the degradation of pyrophosphate, PPi. Indeed, PPi is generated by ectonucleotide pyrophosphatase phosphodiesterase 1 and 3 that are upregulated by 1,25(OH)2D3. The PPi transporter, ANK, is also upregulated while the degradation of PPi is decreased as TNAP is downregulated by 1,25(OH)2D3.15 Each of these effects are mediated by specific vitamin D response elements in the relevant gene promoters.15,45 Other mechanisms controlling mineral deposition such as osteopontin and other SIBLING proteins, as well as their regulating enzymes (such as Phex), are also regulated by 1,25(OH)2D3.38,44,45,46,47 High levels of 1,25(OH)2D3 as found in Vdr−/− pregnant mice can also have deleterious effects on bone mass of their Vdr+/− fetuses by mechanisms still to be fully defined, but one mechanism may be through increased levels of inhibitors of bone mineralization.48
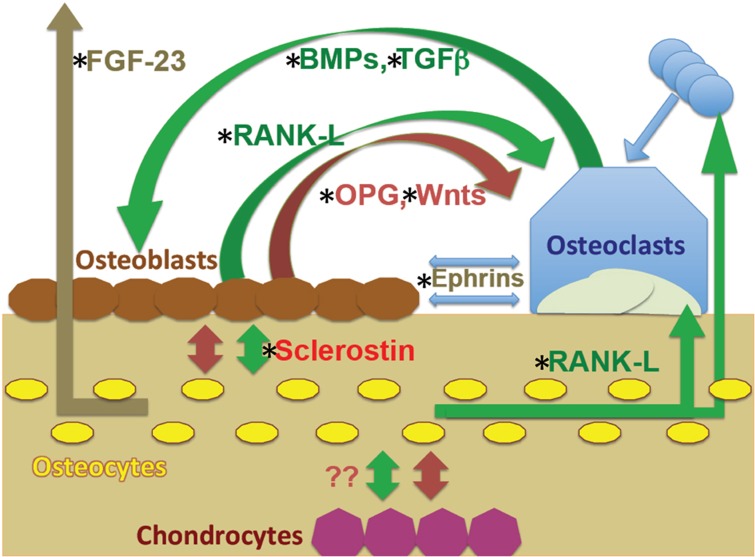
There are thus a large number of independent observations suggesting that the vitamin D endocrine system can have negative direct effects on bone by upregulating bone resorption and by inhibiting of mineral deposition. These effects involve many hormones and humoral factors; all potentially modifiable by 1,25(OH)2D3 (see Figure 1).
Figure 1.
Interactions between cells of the osteoblast and osteoclast lineages, including osteocytes and possible role of chondrocytes. The regulatory pathways between osteoblasts and osteocytes and osteoclasts include multiple negative (red) and positive (green) feedback loops as indicated; all potentially modifiable by 1,25(OH)2D3. These include release of matrix components, such as transforming growth factor-β (TGFβ) and bone morphogenetic proteins (BMPs) that influence osteoblast activity, and humeral factors that influence mineral homeostasis at distant sites, such as fibroblast growth factor (FGF)-23 on renal phosphate handling. Given these loops, any regulatory effect on one cell type can influence the others. Chondrocyte effects on surrounding cells are also potentially involved. The complexity of these inter-relationships may explain the differential effects of 1,25(OH)2D3 in driving anabolic and catabolic outcomes. RANKL, receptor activator of nuclear factor-κB ligand.
A unifying hypothesis on the direct role of vitamin D on bone homeostasis
One way to bring the divergent data on vitamin D and bone into a unifying hypothesis is to look at the evolution of vitamin D in vertebrates (see Bouillon and Suda23 published in first issue (January 2014) of BoneKEy Reports). The vitamin D endocrine system has already been identified in early fish such as lamprey but still without any detectable effect on calcium and certainly not on the still nonexistent mineralized bone. In at least some late teleosts, vitamin D metabolites via VDR probably stimulate a positive calcium balance (possibly more in gills than in the intestine with TRPV channels as mediator). This seems logical as to allow the building up of a large bone structure (mainly acellular, thus without osteocytes and without multinuclear osteoclasts). This is apparent in all amphibians tested, and such an evolution would be logical as terrestrial animals face a calcium-poor environment. So from this early stage in the evolution of vertebrates onwards, the role of vitamin D would be to enhance a positive external calcium balance. From amphibians onwards, PTH becomes a calcium-regulating hormone using bone as a calcium reservoir in times of shortage of nutritional calcium. Whether the vitamin D hormone is already cooperative with PTH to stimulate osteoclastogenesis and bone resorption in amphibians is plausible but needs further exploration. From reptiles onwards, a new dimension is added: a complex system is developing to regulate bone mineral deposition, with hormonal and local regulation of SIBBLING proteins, several enzymes such as PHEX and a complex regulation of pyrophosphate PPi. Most of these factors are under the control of 1,25(OH)2D3 and have, as net result, an inhibition of mineralization. This is not illogical as high 1,25(OH)2D3 with PTH represents the first-line defense against a low nutritional calcium supply by increasing both fractional intestinal absorption and renal calcium reabsorption. Enhanced bone resorption and simultaneous inhibition of mineral deposition can thereby avoid a futile cycle of calcium resorption from bone and its immediate reuse for mineralization of bone.
Therefore, in the first place, the effect of vitamin D action would be to defend systemic calcium homeostasis by making calcium available for the extracellular fluid pool from the intestine if possible but from any internal source if required. In case of very low external calcium supply, high levels of 1,25(OH)2D3 would use the bone calcium reservoir for serum calcium homeostasis at the (temporary) expense of bone mass and strength. From a teleological standpoint, this seems a logical strategy as later access to nutritional calcium could then allow the rebuilding of the skeleton. Thus 1,25(OH)2D3 acting through the VDR may favor replication and maintenance of immature cells of the osteoblast lineage. These roles may be critical for bone resorption and ‘preventing' futile bone mineralization under conditions of calcium stress. On the other hand, during a ‘recovery' phase, 1,25(OH)2D3 acting through the VDR acting in more mature cells may encourage terminal differentiation of mineralizing cells. Consistent with studies of action of 1,25(OH)2D3 on osteoblastic cells at different stages of their maturation,49 this may provide a drive to re-mineralization of the skeleton after surviving the calcium stress situation. This divergence between sacrifice of bone for calcium homoeostasis and rebuilding of the skeleton at other times is not dissimilar from the catabolic effect of chronically high PTH levels versus the anabolic effect of transiently high PTH levels.
Phosphate homeostasis is quite different from that of calcium as terrestrial animals live in a relatively phosphate-rich environment in comparison with marine animals. A phosphaturic hormone (or hormones) may therefore be more important than hormones stimulating phosphate uptake in the intestine or kidney. Although 1,25(OH)2D3 clearly stimulates phosphate uptake in the gut, the VDR endocrine system in the intestine seems to be redundant as selective Vdr−/− in the intestine does not modify serum or urinary phosphate concentration and therefore such animals do not show the typical growth plate abnormalities seen in vitamin D, calcium or phosphate deficiency.
That vitamin D has both beneficial and deleterious effects on bone is not an exception for ligands of nuclear receptors, such as estrogens, androgens or glucocorticoids. In fact, for each of these nuclear receptors it has allowed the development of structural analogs of the natural hormone that have more beneficial and less deleterious effects (for example, selective estrogen receptor modulators). Whether this has already been achieved is further discussed in other chapters of the present special issue (see DeLuca50; Matsutomoto et al.51).
Summary
Global and tissue-specific VDR and CYP27B1 transgenic mice models generate a wealth of data to define the specific role of the vitamin D endocrine system for calcium, phosphate and bone homeostasis.
Overall, these studies confirm that the primary role of the vitamin D pathway in mammals and humans is to stimulate intestinal calcium absorption and to make this ion available for mineralization of bone. This can largely be achieved without direct effects of vitamin D metabolites on bone cells, but there is evidence that the 1,25(OH)2D3–VDR system can drive positive effects on mature osteoblasts so as to facilitate the bone balance if mineral supply is sufficient. Under conditions of calcium stress/deficiency, the vitamin D hormone will, in concert with PTH, defend a normal serum calcium homeostasis, irrespective of any adverse effects on the skeleton. In such situations, vitamin D metabolites can be catabolic, driving bone resorption and decreasing mineral deposition so as to support circulating calcium levels, a clear survival mechanism, analogous to the effect of chronically versus transiently high PTH. Therefore the vitamin D endocrine system has long-term beneficial effects on bone but may transiently use bone as a calcium reservoir for serum calcium homeostasis. Similarly, it may have distinct roles during the ‘recovery' process. This may relate to the 1,25(OH)2D3–VDR system having different functional roles in relation to the stage of differentiation/maturity of the osteoblastic lineage cells, the age of the animals, the external situation, such as calcium or phosphate deficiency or excess, and possibly during fracture repair.
Acknowledgments
We thank Erik Van Herck for technical assistance.
Footnotes
The authors declare no conflict of interest.
References
- Suda T, Takahashi F, Takahashi N. Bone effects of vitamin D—discrepancies between in vivo and in vitro studies. Arch Biochem Biophys 2012;523:22–29. [DOI] [PubMed] [Google Scholar]
- Van Driel M, van Leeuwen JPTM. Vitamin D endocrine system and osteoblasts. BoneKEy Reports 3, Article number: 493 (2014); 10.1038/bonekey.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn 2002;224:245–251. [DOI] [PubMed] [Google Scholar]
- Sims NA, White CP, Sunn KL, Thomas GP, Drummond ML, Morrison NA et al. Human and murine osteocalcin gene expression: conserved tissue restricted expression and divergent responses to 1,25-dihydroxyvitamin D3 in vivo. Mol Endocrinol 1997;11:1695–1708. [DOI] [PubMed] [Google Scholar]
- Demay MB. Physiological insights from the vitamin D receptor knockout mouse. Calcif Tissue Int 2013;92:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon B, Lieben L, Mathieu C, Verstuyf A, Carmeliet G. Vitamin D action: lessons from VDR and Cyp27b1 null mice. Pediatric Rev Endocrinol 2013;10(Suppl 2): 354–366. [PubMed] [Google Scholar]
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 1999;140:4982–4987. [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 1997;94:9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 2001;98:13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R et al. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol 2002;16:1524–1537. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997;16:391–396. [DOI] [PubMed] [Google Scholar]
- Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 1998;139:4391–4396. [DOI] [PubMed] [Google Scholar]
- Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 2009;136:e1311–e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralisation. J Clin Invest 2012;122:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieben L, Carmeliet G, Masuyama R. Calcemic actions of vitamin D: effects on the intestine, kidney and bone. Best Pract Res Clin Endocrinol Metab 2011;25:561–572. [DOI] [PubMed] [Google Scholar]
- Lieben L, Masuyama R, Moermans K, Bouillon R, Carmeliet G. Intestinal-specific vitamin D receptor null mice maintain normal calcemia but display severe bone loss. J Bone Miner Res 2008;23(Suppl 1) (Available at: http://www.abstractsonline.com/viewer/viewAbstract.asp?CKey={270B6864-0501-4492-8638-BD8B56888A99}&MKey={DCB70C83-5B38-431A-B0E1-9221D66718D0}&AKey={D0C01D4F-E23B-45E2-ACD4-0AF8AC866B8B}&SKey={C3DBB86A-71D1-44B3-8B29-91A984611970}). [Google Scholar]
- St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 2000;141:2658–2666. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R, Naja RP. Vitamin D metabolism, cartilage and bone fracture repair. Mol Cell Endocrinol 2011;347:48–54. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA 2001;98:7498–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne O, Prud'homme J, Arabian A, Glorieux F H, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology 2001;142:3135–3141. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 2004;279:16754–16766. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Suda T. Vitamin D: calcium and bone homeostasis during evolution. BoneKEy Reports 3, Article number: 480 (2014); 10.1038/bonekey.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettifor JM, Prentice A. The role of vitamin D in paediatric bone health. Best Pract Res Clin Endocrinol Metab 2011;25:573–584. [DOI] [PubMed] [Google Scholar]
- Mellanby E. An experimental investigation on rickets. Lancet 1919;1:407. [DOI] [PubMed] [Google Scholar]
- Gardiner EM, Baldock PA, Thomas GP, Sims NA, Henderson NK, Hollis B et al. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J 2000;14:1908–1916. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O'Loughlin PD et al. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res 2006;21:1618–1626. [DOI] [PubMed] [Google Scholar]
- Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology 2013;154:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti S, Canalis E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif Tissue Int 2012;90:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 2008;14:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooy K, Sabbagh Y, Demay MB. Osteoblasts lacking the vitamin D receptor display enhanced osteogenic potential in vitro. J Cell Biochem 2005;94:81–87. [DOI] [PubMed] [Google Scholar]
- Yang D, Atkins G, Turner A, Anderson P, Morris H. Differential effects of 1,25-dihydroxyvitamin D on in vitro mineral deposition: interaction between osteoblast stage of maturation and culture medium calcium concentration. J Bone Miner Res 2012;27:SA0222. [Google Scholar]
- Tanaka H, Seino Y. Direct action of 1,25-dihydroxyvitamin D on bone: VDRKO bone shows excessive bone formation in normal mineral condition. J Steroid Biochem Mol Biol 2004;89-90:343–345. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yoshizawa T, Fukuda T, Shirode-Fukuda Y, Yu T, Sekine K et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 2013;154:1008–1020. [DOI] [PubMed] [Google Scholar]
- Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 2006;116:3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naja RP, Dardenne O, Arabian A, St Arnaud R. Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology 2009;150:4024–4032. [DOI] [PubMed] [Google Scholar]
- Lin R, Amizuka N, Sasaki T, Aarts MM, Ozawa H, Goltzman D et al. 1Alpha,25-dihydroxyvitamin D3 promotes vascularization of the chondro-osseous junction by stimulating expression of vascular endothelial growth factor and matrix metalloproteinase 9. J Bone Miner Res 2002;17:1604–1612. [DOI] [PubMed] [Google Scholar]
- Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: effects on bone and mineral homeostasis. Bone 2013;54:237–243. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys 2008;473:225–230. [DOI] [PubMed] [Google Scholar]
- Morrison NA, Shine J, Fragonas JC, Verkest V, McMenemy ML, Eisman JA. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science 1989;246:1158–1161. [DOI] [PubMed] [Google Scholar]
- Terpening CM, Haussler CA, Jurutka PW, Galligan MA, Komm BS, Haussler MR. The vitamin D-responsive element in the rat bone Gla protein gene is an imperfect direct repeat that cooperates with other cis-elements in 1,25-dihydroxyvitamin D3- mediated transcriptional activation. Mol Endocrinol 1991;5:373–385. [DOI] [PubMed] [Google Scholar]
- Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 regulates the expression of low-density lipoprotein receptor-related protein 5 via deoxyribonucleic acid sequence elements located downstream of the start site of transcription. Mol Endocrinol 2006;20:2215–2230. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Weitzmann MN. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int J Mol Med 2012;29:934–938. [DOI] [PubMed] [Google Scholar]
- Bouillon R. Report on recent vitamin D research: ECTS 2012 and the 15th Vitamin D workshop 2012. IBMS BoneKEy 9: article number 203 (2012); 10.1038/bonekey.2012.203 [DOI] [Google Scholar]
- Meyer MB, Lee CH, Benkusky NA, Sen B, Rubin J, Pike JW. 15th Vitamin D Workshop Abstract Book 27:University of California: Riverside, CA, USA, 2012;. [Google Scholar]
- Rowe PS. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem Funct 2012;30:355–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr 2012;22:61–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieben L, Stockmans I, Moermans K, Carmeliet G. Maternal hypervitaminosis D reduces fetal bone mass and mineral acquisition and leads to neonatal lethality. Bone 2013;57:123–131. [DOI] [PubMed] [Google Scholar]
- Shi YC, Worton L, Esteban L, Baldock P, Fong C, Eisman JA et al. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone 2007;41:87–96. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. The development of bone- and parathyroid-specific analog of Vitamin D: 2-methylene-19-nor-(20S)-1α,25-dihydroxyvitamin D3. BoneKEy Reports 3, Article number: 514 (2014); 10.1038/bonekey.2014.9 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumo T, Takano T, Saito H, Takahashi F. Vitamin D analogs and bone: preclinical and clinical studies with eldecalcitol. BoneKEy Reports 3, Article number: 513 (2014); 10.1038/bonekey.2014.8 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]