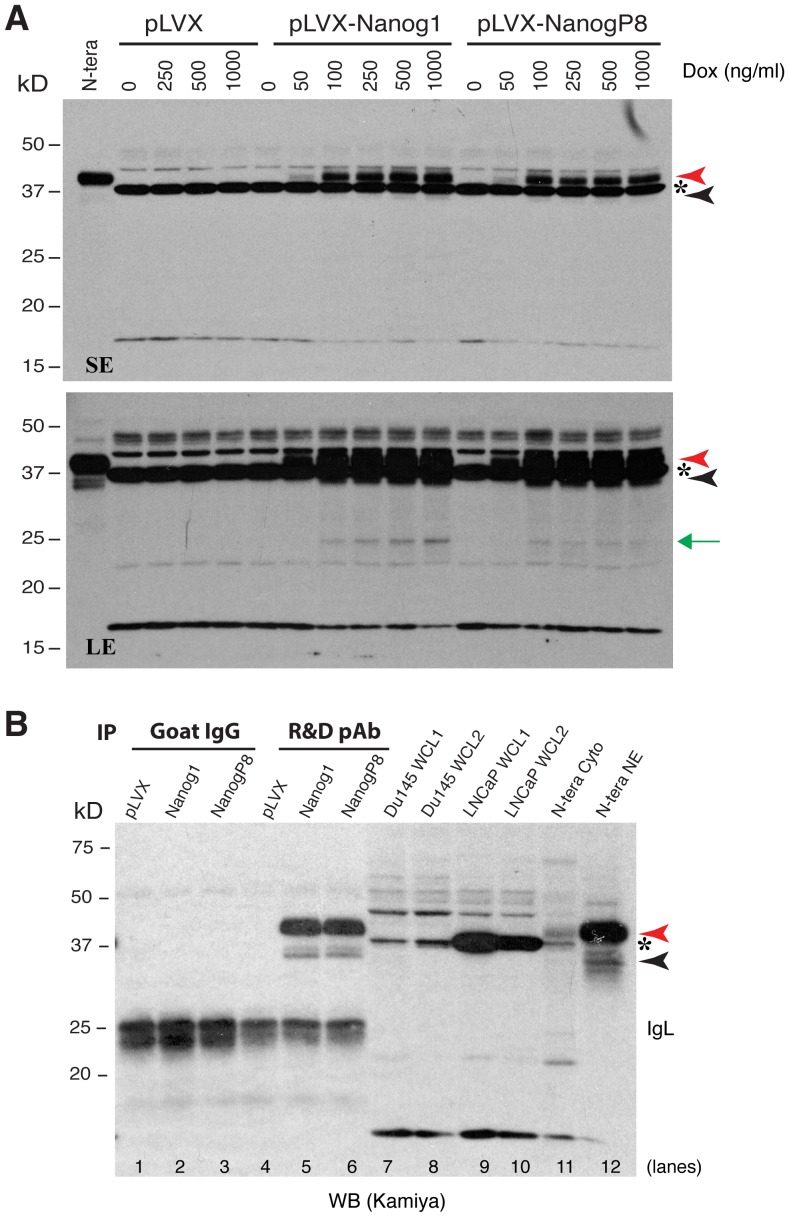
Figure 5. Exogenous NanogP8 expressed in LNCaP cells migrates mainly at 42 kD.
(A) Exogenous NanogP8 migrates at 42 kD on WB. Whole cell lysate (80 µg/lane) prepared from control LNCaP (pLVX) or Nanog1/NanogP8 overexpressing LNCaP cells [34] in the presence of increasing amounts of doxycycline (Dox) was used in WB with the Kamiya anti-Nanog rabbit pAb. The red and black arrowheads indicate the main 42 kD and minor 35 kD Nanog bands, respectively. Green arrow, a ∼28 kD band that also increased upon Dox induction. Asterisk, a non-specific band. N-tera, NE of NTERA-2 cell; SE, short exposure; LE, long exposure. (B) The exogenous 42 kD and 35 kD bands could be immunoprecipitated down by the R&D anti-Nanog pAb. Whole-cell lysate (WCL; 500 µg) derived from pLVX, pLVX-NANOG1 and pLVX-NANOGP8 LNCaP cells [34] were used in IP with the R&D anti-Nanog goat pAb (goat IgG used as the control) followed by WB with Kamiya anti-Nanog rabbit pAb. The red and black arrowheads indicate the main 42 kD and minor 35 kD Nanog bands, respectively. Asterisk, a non-specific band. NE, nuclear extract; cyto, cytosol protein. Note that the 42-kD and the 35-kD protein bands only from pLVX-NANOG1 and pLVX-NANOGP8 LNCaP cells (but not from LNCaP-pLVX cells) were IP'ed down and detected on WB (lanes 5,6). Goat IgG did not pull down any specific bands. Also, WCL from two batches (1 and 2) of Du145 s and LNCaP cells (80 µg/lane) did not reveal the 42 kD and 35 kD protein bands on WB (lanes 7-10). The 37 kD non-specific band (asterisk) was not immunoprecipitated down by the R&D goat pAb (lane 5–6).