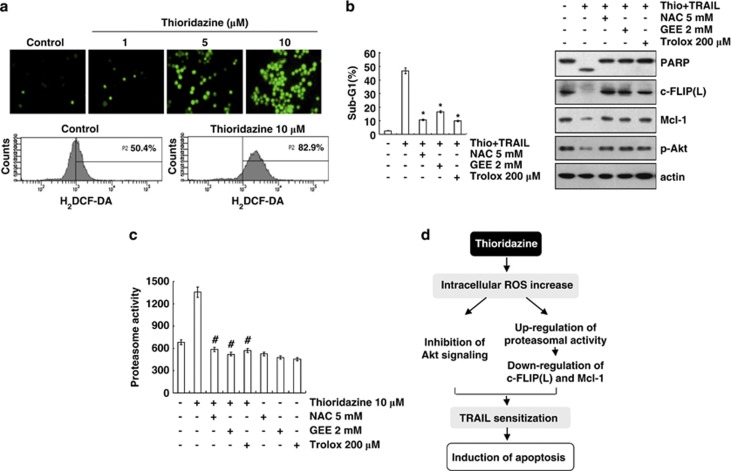
Figure 6.
Thioridazine plus TRAIL-induced apoptosis is dependent on ROS generation in Caki cells. (a) Caki cells were treated with the indicated concentrations of thioridazine for 30 min and loaded with a H2DCFDA fluorescent dye. H2DCFDA fluorescence intensity was detected by fluorescence microscopy (upper panel) and flow cytometry (lower panel). (b) Caki cells were pretreated with 5 mM NAC, 2 mM GEE, and 200 μM trolox for 30 min, and then stimulated with 10 μM thioridazine (thio) and 50 ng/ml TRAIL for 24 h. Apoptosis was analyzed as the sub-G1 population by FACS analysis. The protein expression levels of PARP, c-FLIP(L), Mcl-1, phospho (p)-Akt, and actin were determined by western blotting. The level of actin was used as a loading control. *P<0.001 compared with thioridazine plus TRAIL. (c) Caki cells were pretreated with 5 mM NAC, 2 mM GEE, 200 μM trolox for 30 min, and then stimulated with 10 μM thioridazine for 24 h. The cells were lysed, and proteasome activity was measured as described in the Materials and Methods section. #P<0.001 compared with thioridazine. (d) Models for thioridazine-mediated TRAIL sensitization in Caki cells. Thioridazine triggers intracellular ROS production. Increased ROS inhibit Akt signaling and increase proteasome activity. Upregulation of proteasome activity leads to the downregulation of c-FLIP(L) and Mcl-1 expression at the post-translational levels. Both inhibitions of Akt signaling and upregulation of proteasome activity are associated with induction of TRAIL-mediated apoptosis in thioridazine-treated Caki cells