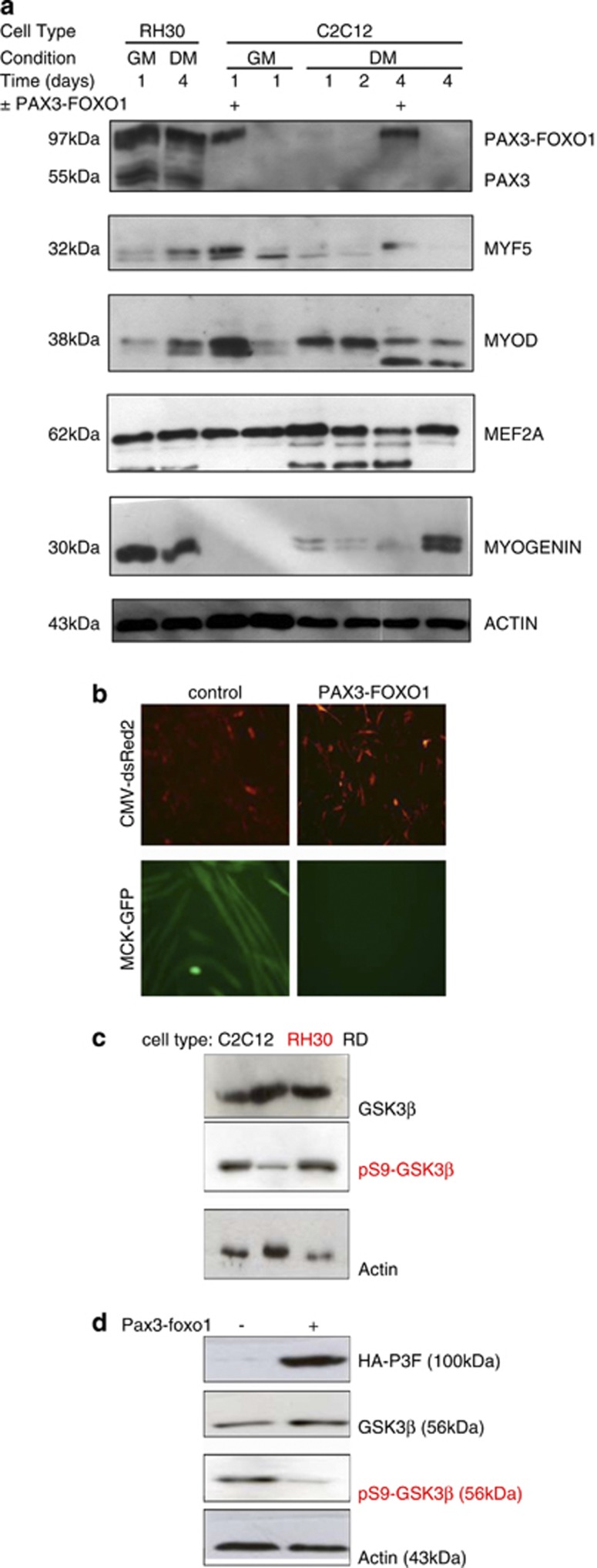
Figure 1.
MYOGENIN protein expression and GSK3β activity are both maintained in ARMS: (a) C2C12 myoblasts were transfected with HA-PAX3-FOXO1 or pcDNA3.1 control plasmid for 1 day before extraction or serum withdrawal and then extraction at 1 day increments for up to 4 days as indicated. Protein levels were compared with protein extracts from PAX3-FOXO1 expressing RH30 cells 1 day in growth media (GM) and 4 days in differentiation media (DM). The results show that despite the expression of PAX3-FOXO1, RH30 cells also express MYOGENIN. On the other hand, HA-PAX3-FOXO1 overexpression in C2C12 inhibits MYOGENIN expression and subsequent myogenic differentiation. (b) C2C12 myoblasts were transfected with CMV-dsRed2, MCK-eGFP and, either HA-PAX3-FOXO1 or pcDNA3.1 control plasmid. HA-PAX3-FOXO1 overexpression repressed the formation of multinucleated myotubes. (c) Endogenous GSK3β protein levels and phosphorylation at serine 9 were compared in C2C12 myoblasts, RH30 and ERMS RD cells. Although GSK3β is expressed in all three cell types, it is predominantly phosphorylated and hence inactive in C2C12 myoblasts and RD cells but not PAX3-FOXO1 expressing RH30 cells. (d) C2C12 myoblasts were transfected with HA-PAX3-FOXO1 or pcDNA3.1 control plasmid for 1 day before extraction. Overexpression of HA-PAX3-FOXO1 resulted in decreased phosphorylation of GSK3β at serine 9 indicating its activation