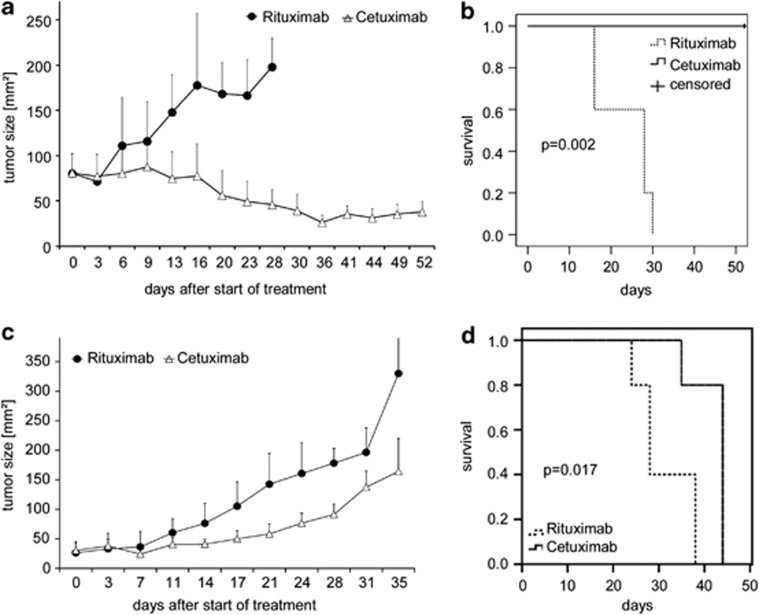
Figure 2.
Effective treatment of established HPV-negative and HPV-positive tumors by treatment with cetuximab in vivo. Palpable flank tumors were established by subcutaneous injection of (a and b) FaDu (HPV-negative) or (c and d) UPCI:SCC-090 (HPV-positive) cells in NOD/SCID mice. After 14 days, tumor-bearing mice were treated twice weekly by intraperitoneal injections of cetuximab (1 mg, white triangles) or the control antibody rituximab (1 mg, black circles). (a and c) Tumor growth was monitored by palpation and quantified using a caliper. Mean bidimensional tumor sizes (+s.d.) of a representative experiment (five mice per treatment group) are given. (b and d) Kaplan–Meier plots of survival of tumor-bearing NOD/SCID mice. (b) HPV-negative FaDu; (d) HPV-positive UPCI:SCC-090. Mice were treated as in a and c with cetuximab (solid line), or the control antibody rituximab (dashed line). Cetuximab-treated mice exhibited a significantly prolonged survival as compared with rituximab-treated mice. In the FaDu model, median survival time for cetuximab-treated mice was not reached. It was 28 days for mice receiving rituximab (P=0.002, log rank test). In the UPCI:SCC-090 model, median survival time for cetuximab-treated mice was 44 days, and 28 days for rituximab-treated mice (P=0.017, log rank test)