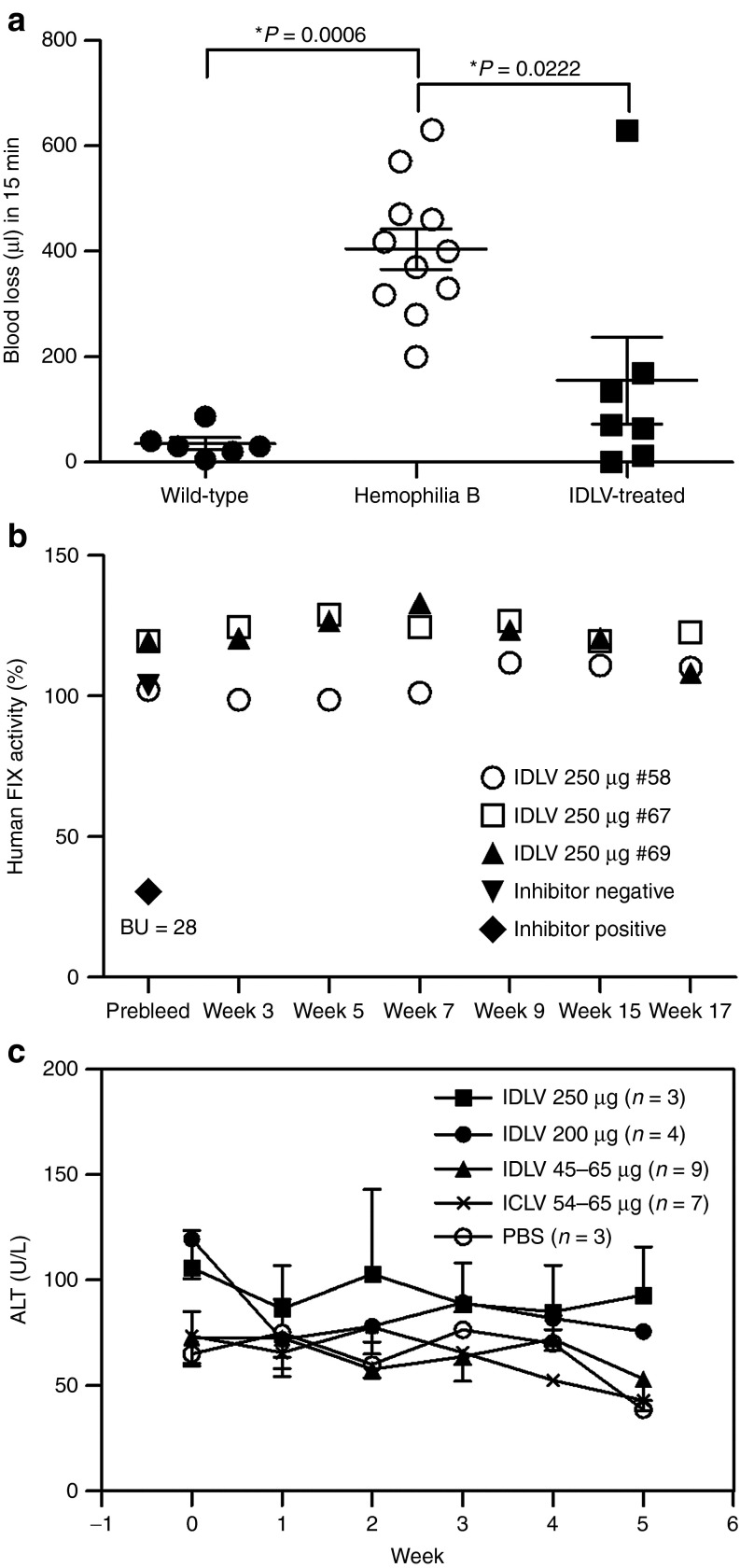
Figure 3.
Integration-deficient lentiviral vector (IDLV) delivery of the codon-optimized R338L human FIX rendered hemophilia B mice resistant to tail-clipping injury without inducing immunologic adverse effects. (a) Functional clotting assay in vivo. Hemophilia B mice were intraperitoneally injected with IDLVs carrying the codon-optimized R338L human FIX cDNA under the control of the human α1-antitrypsin promoter (pTK1340), with doses of either 200 µg (n = 4) or 250 µg (n = 3) p24gag. At 20 weeks postvector administration, treated mice were challenged with a tail-clipping assay. Total volume of blood lost in 15 min was measured in seven vector-treated mice and compared with blood loss of tail-clipped wild-type (n = 6) and phosphate-buffered saline (PBS)-injected hemophilia B mice (n = 11). P value was assessed by a paired Student's t-test. (b) Lack of neutralizing antibodies directed to human FIX protein in IDLV-treated mice. A modified Bethesda assay was used to detect the emergence of inhibitory human FIX antibodies. Plasma samples were collected from the aforementioned IDLV-treated hemophilia B mice (250 µg p24gag, n = 3) before (prebleed) and at various time points postvector administration and were analyzed for the presence of inhibitory antibodies. Plasma samples obtained from a naive (untreated) mouse and from the human FIX inhibitor-producing mouse (a hemophilia B mouse injected with purified human FIX protein and exhibiting 28 Bethesda units (BU)/ml), were used as negative and positive control, respectively. (c) Evaluation of vector-mediated hepatotoxicity. To detect liver damage induced by either uptake of vector particles or due to human FIX production, levels of alanine aminotransferase (ALT) were determined in mouse plasma obtained before and periodically after vector administration. Samples from PBS-treated mice were used as negative controls. ICLV, integrase-competent lentiviral vectors.