Abstract
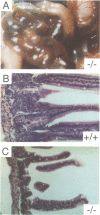
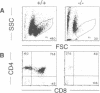
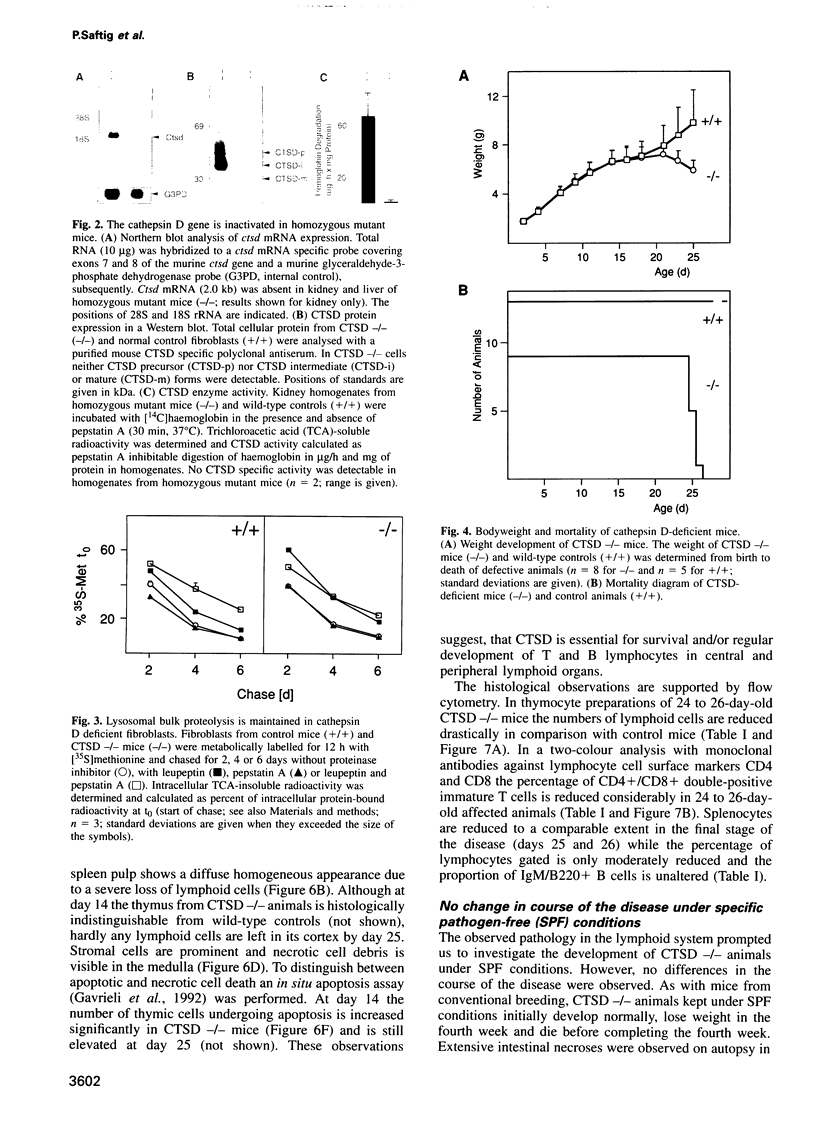
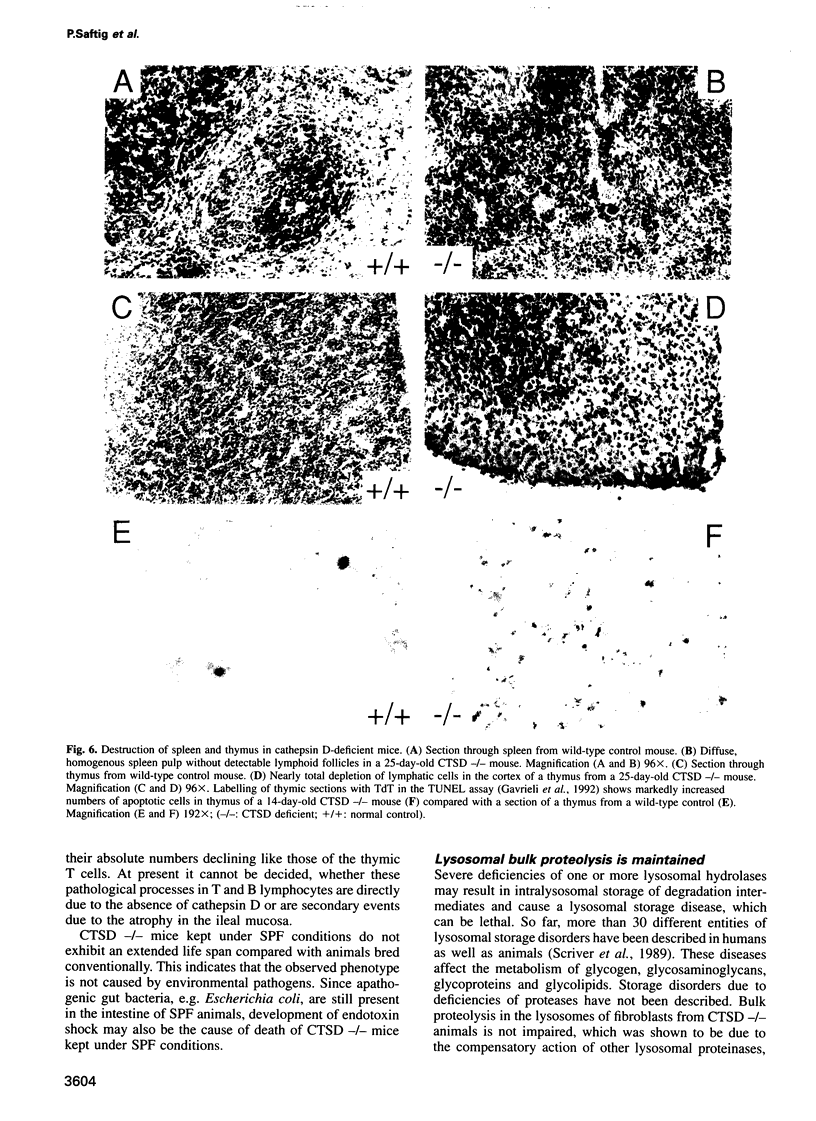
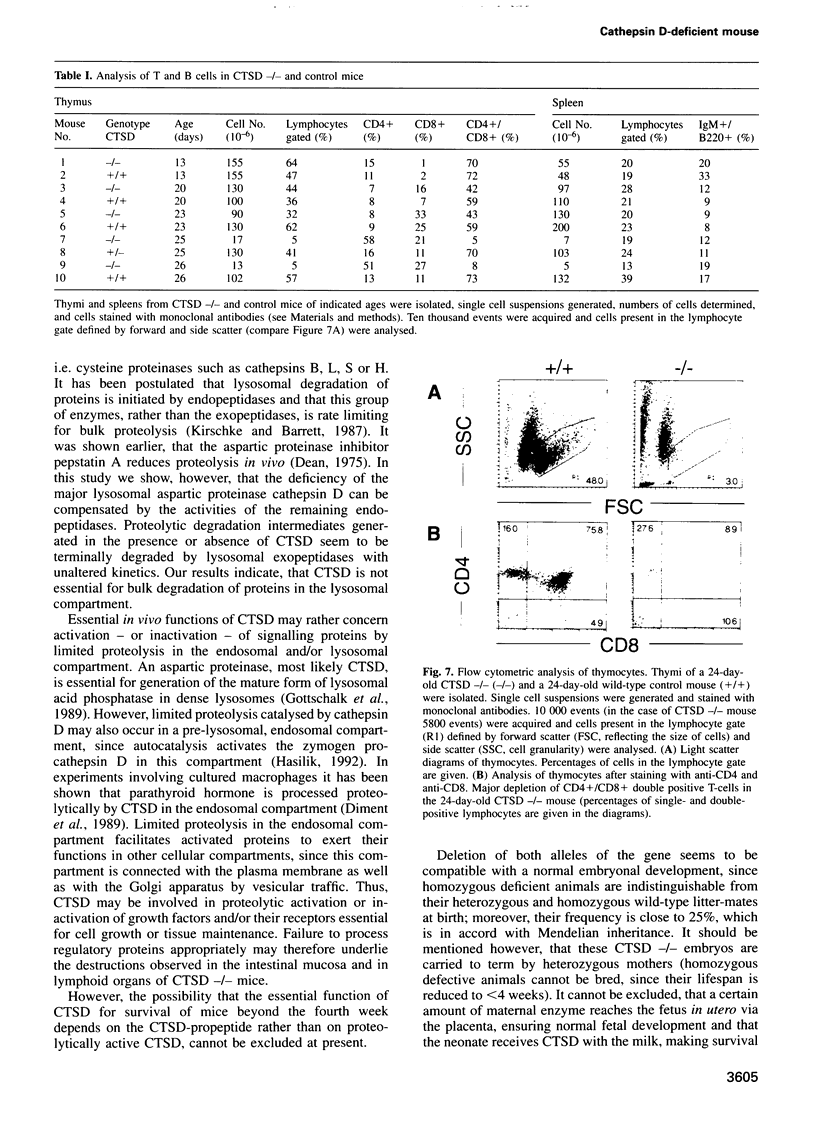
Mice deficient for the major lysosomal aspartic proteinase cathepsin D, generated by gene targeting, develop normally during the first 2 weeks, stop thriving in the third week and die in a state of anorexia at day 26 +/- 1. An atrophy of the ileal mucosa first observed in the third week progresses towards widespread intestinal necroses accompanied by thromboemboli. Thymus and spleen undergo massive destruction with fulminant loss of T and B cells. Lysosomal bulk proteolysis is maintained. These results suggest, that vital functions of cathepsin D are exerted by limited proteolysis of proteins regulating cell growth and/or tissue homeostasis, while its contribution to bulk proteolysis in lysosomes appears to be non-critical.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Cellular proteolysis. An overview. Ann N Y Acad Sci. 1992 Dec 31;674:1–15. doi: 10.1111/j.1749-6632.1992.tb27472.x. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christy J. H. Pathophysiology of gram-negative shock. Am Heart J. 1971 May;81(5):694–701. doi: 10.1016/0002-8703(71)90014-7. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994 Oct 7;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Direct evidence of importance of lysosomes in degradation of intracellular proteins. Nature. 1975 Oct 2;257(5525):414–416. doi: 10.1038/257414a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Diment S., Martin K. J., Stahl P. D. Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J Biol Chem. 1989 Aug 15;264(23):13403–13406. [PubMed] [Google Scholar]
- Diment S., Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985 Dec 5;260(28):15311–15317. [PubMed] [Google Scholar]
- Elin R. J., Wolff S. M. Biology of endotoxin. Annu Rev Med. 1976;27:127–141. doi: 10.1146/annurev.me.27.020176.001015. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993 Apr;187(3-5):346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Hasilik A., von Figura K. Possible pathways for lysosomal enzyme delivery. J Cell Biol. 1985 Dec;101(6):2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S., Waheed A., Schmidt B., Laidler P., von Figura K. Sequential processing of lysosomal acid phosphatase by a cytoplasmic thiol proteinase and a lysosomal aspartyl proteinase. EMBO J. 1989 Nov;8(11):3215–3219. doi: 10.1002/j.1460-2075.1989.tb08480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A. The early and late processing of lysosomal enzymes: proteolysis and compartmentation. Experientia. 1992 Feb 15;48(2):130–151. doi: 10.1007/BF01923507. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Ontogeny of enzymes in the small intestine. Annu Rev Physiol. 1985;47:231–245. doi: 10.1146/annurev.ph.47.030185.001311. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Sunshine P. Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res. 1969 Jan;3(1):27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
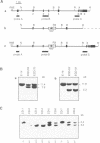
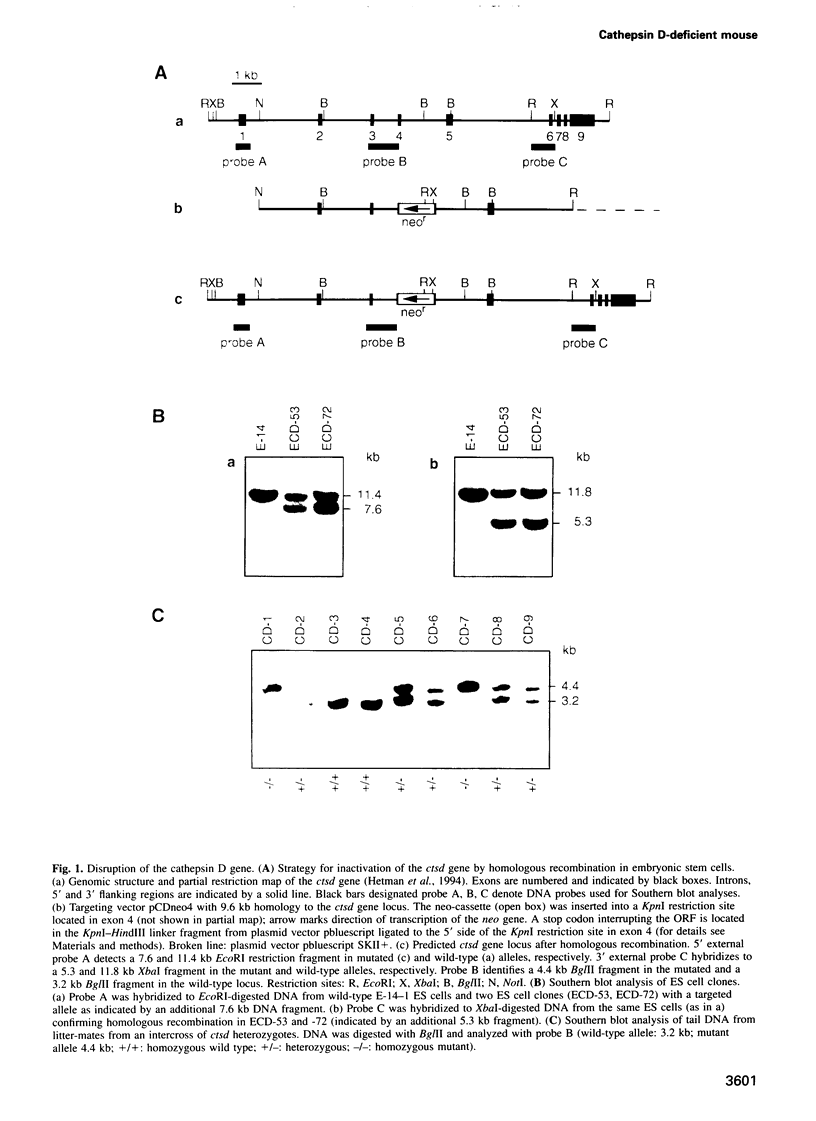
- Hetman M., Perschl A., Saftig P., Von Figura K., Peters C. Mouse cathepsin D gene: molecular organization, characterization of the promoter, and chromosomal localization. DNA Cell Biol. 1994 Apr;13(4):419–427. doi: 10.1089/dna.1994.13.419. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Horst M., Hasilik A. Expression and maturation of human cathepsin D in baby-hamster kidney cells. Biochem J. 1991 Jan 15;273(Pt 2):355–361. doi: 10.1042/bj2730355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrandt D., Arlt G., Brooks D. A., Hopwood J. J., von Figura K., Peters C. Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): six unique arylsulfatase B gene alleles causing variable disease phenotypes. Am J Hum Genet. 1994 Mar;54(3):454–463. [PMC free article] [PubMed] [Google Scholar]
- Koldovsky O., Sunshine P., Kretchmer N. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature. 1966 Dec 17;212(5068):1389–1390. doi: 10.1038/2121389a0. [DOI] [PubMed] [Google Scholar]
- Kopitz J., Arnold A., Meissner T., Cantz M. Protein catabolism in fibroblasts cultured from patients with mucolipidosis II and other lysosomal disorders. Biochem J. 1993 Oct 15;295(Pt 2):577–580. doi: 10.1042/bj2950577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster A., Saftig P., Matzner U., von Figura K., Peters C., Pohlmann R. Targeted disruption of the M(r) 46,000 mannose 6-phosphate receptor gene in mice results in misrouting of lysosomal proteins. EMBO J. 1993 Dec 15;12(13):5219–5223. doi: 10.1002/j.1460-2075.1993.tb06217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Synthesis and transport of lysosomal acid phosphatase in normal and I-cell fibroblasts. J Biol Chem. 1985 Jul 25;260(15):9023–9030. [PubMed] [Google Scholar]
- Leto G., Gebbia N., Rausa L., Tumminello F. M. Cathepsin D in the malignant progression of neoplastic diseases (review). Anticancer Res. 1992 Jan-Feb;12(1):235–240. [PubMed] [Google Scholar]
- Lyons K., Graycar J. L., Lee A., Hashmi S., Lindquist P. B., Chen E. Y., Hogan B. L., Derynck R. Vgr-1, a mammalian gene related to Xenopus Vg-1, is a member of the transforming growth factor beta gene superfamily. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4554–4558. doi: 10.1073/pnas.86.12.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marić M. A., Taylor M. D., Blum J. S. Endosomal aspartic proteinases are required for invariant-chain processing. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2171–2175. doi: 10.1073/pnas.91.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf P., Fusek M. Two crystal structures for cathepsin D: the lysosomal targeting signal and active site. EMBO J. 1993 Apr;12(4):1293–1302. doi: 10.1002/j.1460-2075.1993.tb05774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Intracellular transport of MHC class II molecules. Immunol Today. 1992 May;13(5):179–184. doi: 10.1016/0167-5699(92)90123-O. [DOI] [PubMed] [Google Scholar]
- Offermann M. K., Chlebowski J. F., Bond J. S. Action of cathepsin D on fructose-1,6-bisphosphate aldolase. Biochem J. 1983 Jun 1;211(3):529–534. doi: 10.1042/bj2110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., Schmidt B., Rommerskirch W., Rupp K., Zühlsdorf M., Vingron M., Meyer H. E., Pohlmann R., von Figura K. Phylogenetic conservation of arylsulfatases. cDNA cloning and expression of human arylsulfatase B. J Biol Chem. 1990 Feb 25;265(6):3374–3381. [PubMed] [Google Scholar]
- Poole A. R., Hembry R. M., Dingle J. T. Cathepsin D in cartilage: the immunohistochemical demonstration of extracellular enzyme in normal and pathological conditions. J Cell Sci. 1974 Jan;14(1):139–161. doi: 10.1242/jcs.14.1.139. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Reid W. A., Valler M. J., Kay J. Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol. 1986 Dec;39(12):1323–1330. doi: 10.1136/jcp.39.12.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., Osborne B. A. Programmed cell death, apoptosis and killer genes. Immunol Today. 1993 Dec;14(12):582–590. doi: 10.1016/0167-5699(93)90197-S. [DOI] [PubMed] [Google Scholar]
- Tandon A. K., Clark G. M., Chamness G. C., Chirgwin J. M., McGuire W. L. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990 Feb 1;322(5):297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- Tang J. Evolution in the structure and function of carboxyl proteases. Mol Cell Biochem. 1979 Jul 31;26(2):93–109. doi: 10.1007/BF00232887. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Wenk J., Hille A., von Figura K. Quantitation of Mr 46000 and Mr 300000 mannose 6-phosphate receptors in human cells and tissues. Biochem Int. 1991 Mar;23(4):723–731. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- van Noort J. M., Jacobs M. J. Cathepsin D, but not cathepsin B, releases T cell stimulatory fragments from lysozyme that are functional in the context of multiple murine class II MHC molecules. Eur J Immunol. 1994 Sep;24(9):2175–2180. doi: 10.1002/eji.1830240936. [DOI] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]