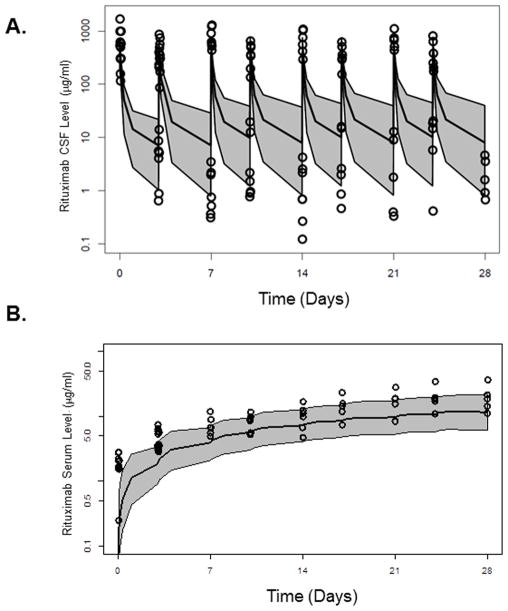
Figure 5.
Figure 5A and 5B. Rituximab concentration-time profiles in (5A) CSF and (5B) serum from 7 patients in 25 mg dose group from second phase I trial of intraventricular rituximab. Overall disposition of rituximab in the second study was successfully predicted based upon the three compartment model derived from analysis of rituximab pharmacokinetics in the first phase I trial of intraventricular rituximab. Open circles represent the original observations; shaded area represents the 95% prediction internal according to the model; the black lines represent the 97.5, 50, and 2.5th percentiles of the simulated data.