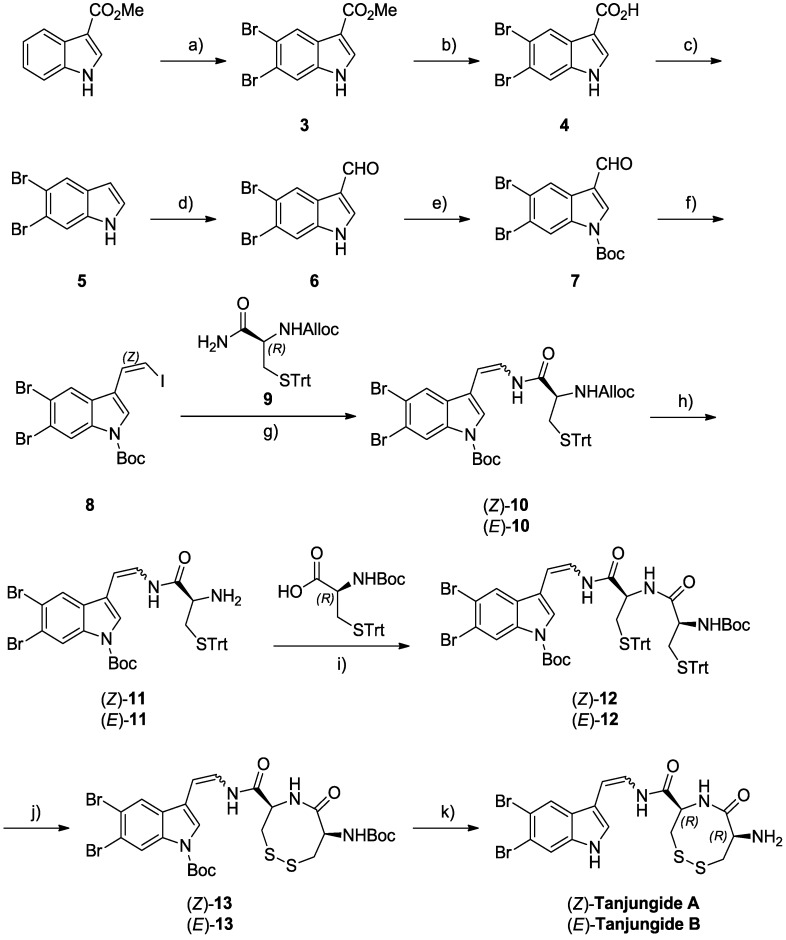
Scheme 1.
Total synthesis of Tanjungides A (1) and B (2).
Regents and conditions: (a) Br2, AcOH, 23 °C, 2 h, 66%; (b) aq NaOH 2 M, CH3OH, reflux, 2.5 h, 95%; (c) Pyridine, reflux, 12 h, 95%; (d) i POCl3, DMF, 35 °C, 1 h then 65 °C, 1 h, ii aq NaOH 2 M, 110 °C, 5 min, 92%; (e) (Boc)2O, DMAP, 1,4-Dioxane, 23 °C, 2 h, 86%; (f) Iodomethyltriphpenylphosphonium iodide, NaHMDS 1.0 M, THF, −78 °C, 2 h, 83%; (g) for (Z)-10: 9, Cul, Cs2CO3, DMEDA, THF, 60 °C, 18 h, 50% + 13% (E)-10; for (E)-10: 9, Cul, K2CO3, DMEDA, THF, 80 °C, 18 h, 60% + 14% (Z)-10; (h) Pd(PPh3)4, PhSiH3, CH2Cl2, 23 °C, 30 min, 85% (Z)-11 and 72% (E)-11; (i) N-Boc-l-(S-trityl)-Cys, HATU, HOBt, DIPEA, CH2Cl2:DMF (4:1), 23 °C, 2 h, 83% (Z)-12 and 62% (E)-12; (j) I2, CH2Cl2:CH3OH (10:1), 23 °C, 40 min, 84% (Z)-13 and 73% (E)-13; (k) TFA, CH2Cl2, 0 °C, 3 h, 60% (Z)-Tanjungide A and 45% (E)-Tanjungide B.