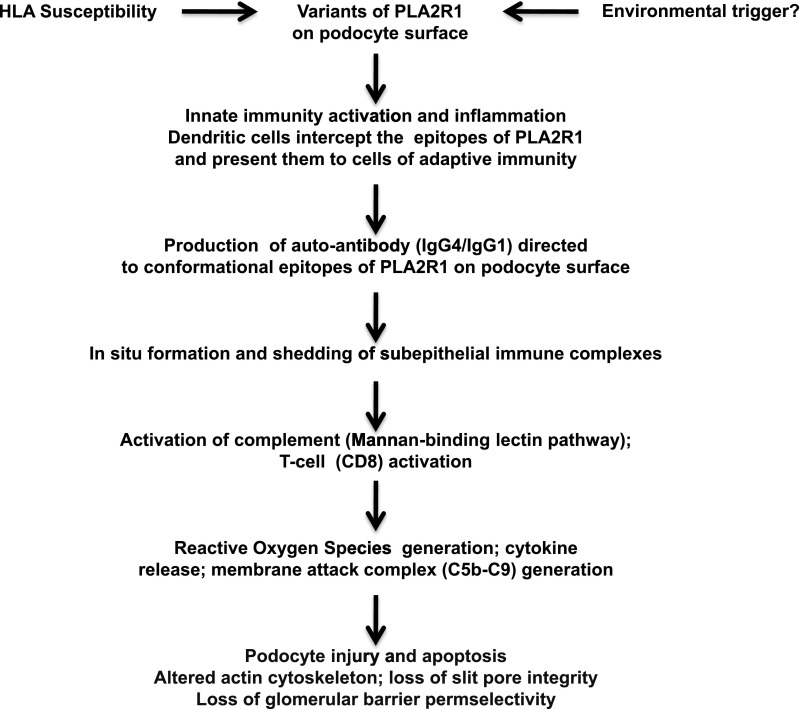
Figure 1.
A personal hypothetical mechanistic interpretation of idiopathic MN. In genetically predisposed patients, a polymorphism of PLA2R1 may create a unique conformational variant. Alternatively endogenous or exogenous injuries (viral attack?) may cause structural alterations of PLA2R1 or lead to podocyte injury with exposure of some “hidden” epitopes of PLA2R1. It is possible that these abnormal molecular patterns are recognized as danger signals by the receptors of innate immunity with consequent recruitment of inflammatory cells. In this inflammatory microenvironment, the dendritic cells would become mature, intercept the epitopes of PLA2R1 expressed on the glomerular podocyte surface, and migrate to lymph nodes where they present the antigen to the cells of the adaptive immunity. The B cells would differentiate into plasma cells that secrete specific hypogalactosylated IgG4. These antibodies attack the epitopes of PLA2R1 on podocyte surface, with formation of subepithelial immune complexes and consequent production of ROS, and inflammation. The hypogalactosylated IgG4 activates the complement system through the lectin pathway which initiates the complement cascade, with the final production of the cytolytic C5b-C9 complex, also called the membrane attack complex. As a consequence, there is an increased damage of podocytes, alteration of the actin cytoskeleton, loss of the glomerular barrier permselectivity, and proteinuria. MN, membranous nephropathy; PLA2R1, phospholipase A2 receptor 1; ROS, reactive oxygen species.