Abstract
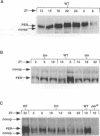
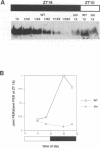
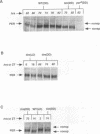
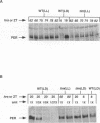
The timeless mutation (tim) leads to loss of circadian behavioral rhythms in Drosophila melanogaster. The effects of tim on rhythmicity involve interactions with period (per), a second essential clock gene, as the tim mutation suppresses circadian oscillations of per transcription and blocks nuclear localization of a PER reporter protein. In the present study it was found that the tim mutant constitutively produces a low level of PER protein that is comparable with that produced late in the day by wild-type flies. In addition, it was shown that tim suppresses circadian cycling of PER protein abundance and circadian regulation of PER phosphorylation. Transfer of wild-type flies to constant light also suppressed cycling of PER abundance and phosphorylation and produced constitutively low levels of PER. In the tim mutant there was no additional effect of constant light on PER. These results suggest that constant light and the tim mutation produce related changes in the underlying biological clock. We further suggest that the multiple effects of tim are due to a primary effect on per expression at the posttranscriptional level. The effects of tim on behavioral rhythms and per RNA cycling are therefore likely to involve effects on PER protein through previously proposed feedback mechanisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson B. D., Johnson K. A., Loros J. J., Dunlap J. C. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994 Mar 18;263(5153):1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- Bruce V. G. Mutants of the biological clock in Chlamydomonas reinhardi. Genetics. 1972 Apr;70(4):537–548. doi: 10.1093/genetics/70.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S. T., Thomas J. B., Goodman C. S. The Drosophila single-minded gene encodes a nuclear protein with sequence similarity to the per gene product. Cell. 1988 Jan 15;52(1):143–151. doi: 10.1016/0092-8674(88)90538-7. [DOI] [PubMed] [Google Scholar]
- Curtin K. D., Huang Z. J., Rosbash M. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron. 1995 Feb;14(2):365–372. doi: 10.1016/0896-6273(95)90292-9. [DOI] [PubMed] [Google Scholar]
- Edery I., Zwiebel L. J., Dembinska M. E., Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Hoyle M. N. Isolation of circadian clock mutants of Neurospora crassa. Genetics. 1973 Dec;75(4):605–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässer F. A., König S. Phosphorylation of SV40 large T antigen at threonine residues results in conversion to a lower apparent molecular weight form. Arch Virol. 1992;126(1-4):313–320. doi: 10.1007/BF01309704. [DOI] [PubMed] [Google Scholar]
- Hall J. C. Genetics of circadian rhythms. Annu Rev Genet. 1990;24:659–697. doi: 10.1146/annurev.ge.24.120190.003303. [DOI] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Behavioral and molecular analyses suggest that circadian output is disrupted by disconnected mutants in D. melanogaster. EMBO J. 1992 Jan;11(1):1–6. doi: 10.1002/j.1460-2075.1992.tb05020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990 Feb 8;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- King R. W., Jackson P. K., Kirschner M. W. Mitosis in transition. Cell. 1994 Nov 18;79(4):563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kondo T., Tsinoremas N. F., Golden S. S., Johnson C. H., Kutsuna S., Ishiura M. Circadian clock mutants of cyanobacteria. Science. 1994 Nov 18;266(5188):1233–1236. doi: 10.1126/science.7973706. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R. J., Pittendrigh C., Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet. 1989 Sep;6(1):1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- Liu X., Lorenz L., Yu Q. N., Hall J. C., Rosbash M. Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev. 1988 Feb;2(2):228–238. doi: 10.1101/gad.2.2.228. [DOI] [PubMed] [Google Scholar]
- Liu X., Zwiebel L. J., Hinton D., Benzer S., Hall J. C., Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992 Jul;12(7):2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita N., Sogawa K., Ema M., Yoshida A., Fujii-Kuriyama Y. A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt. J Biol Chem. 1993 Oct 5;268(28):21002–21006. [PubMed] [Google Scholar]
- McKinney J. D., Chang F., Heintz N., Cross F. R. Negative regulation of FAR1 at the Start of the yeast cell cycle. Genes Dev. 1993 May;7(5):833–843. doi: 10.1101/gad.7.5.833. [DOI] [PubMed] [Google Scholar]
- Millar A. J., Carré I. A., Strayer C. A., Chua N. H., Kay S. A. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science. 1995 Feb 24;267(5201):1161–1163. doi: 10.1126/science.7855595. [DOI] [PubMed] [Google Scholar]
- Moll T., Tebb G., Surana U., Robitsch H., Nasmyth K. The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell. 1991 Aug 23;66(4):743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- Petersen G., Hall J. C., Rosbash M. The period gene of Drosophila carries species-specific behavioral instructions. EMBO J. 1988 Dec 1;7(12):3939–3947. doi: 10.1002/j.1460-2075.1988.tb03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph M. R., Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988 Sep 2;241(4870):1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Hall J. C. The molecular biology of circadian rhythms. Neuron. 1989 Oct;3(4):387–398. doi: 10.1016/0896-6273(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Sehgal A., Price J. L., Man B., Young M. W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994 Mar 18;263(5153):1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Shuai K., Horvath C. M., Huang L. H., Qureshi S. A., Cowburn D., Darnell J. E., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994 Mar 11;76(5):821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Siwicki K. K., Eastman C., Petersen G., Rosbash M., Hall J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988 Apr;1(2):141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Traenckner E. B., Wilk S., Baeuerle P. A. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994 Nov 15;13(22):5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna M. H., King D. P., Chang A. M., Kornhauser J. M., Lowrey P. L., McDonald J. D., Dove W. F., Pinto L. H., Turek F. W., Takahashi J. S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994 Apr 29;264(5159):719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall L. B., Price J. L., Sehgal A., Saez L., Young M. W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994 Mar 18;263(5153):1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- Zeng H., Hardin P. E., Rosbash M. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 1994 Aug 1;13(15):3590–3598. doi: 10.1002/j.1460-2075.1994.tb06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr D. M., Hall J. C., Rosbash M., Siwicki K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990 Aug;10(8):2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]