ABSTRACT
Induction of immunity that limits Toxoplasma gondii infection in mice is critically dependent on the activation of the innate immune response. In this study, we investigated the role of cytoplasmic nucleotide-binding domain and leucine-rich repeat containing a pyrin domain (NLRP) inflammasome sensors during acute toxoplasmosis in mice. We show that in vitro Toxoplasma infection of murine bone marrow-derived macrophages activates the NLRP3 inflammasome, resulting in the rapid production and cleavage of interleukin-1β (IL-1β), with no measurable cleavage of IL-18 and no pyroptosis. Paradoxically, Toxoplasma-infected mice produced large quantities of IL-18 but had no measurable IL-1β in their serum. Infection of mice deficient in NLRP3, caspase-1/11, IL-1R, or the inflammasome adaptor protein ASC led to decreased levels of circulating IL-18, increased parasite replication, and death. Interestingly, mice deficient in NLRP1 also displayed increased parasite loads and acute mortality. Using mice deficient in IL-18 and IL-18R, we show that this cytokine plays an important role in limiting parasite replication to promote murine survival. Our findings reveal T. gondii as a novel activator of the NLRP1 and NLRP3 inflammasomes in vivo and establish a role for these sensors in host resistance to toxoplasmosis.
IMPORTANCE
Inflammasomes are multiprotein complexes that are a major component of the innate immune system. They contain “sensor” proteins that are responsible for detecting various microbial and environmental danger signals and function by activating caspase-1, an enzyme that mediates cleavage and release of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18. Toxoplasma gondii is a highly successful protozoan parasite capable of infecting a wide range of host species that have variable levels of resistance. We report here that T. gondii is a novel activator of the NLRP1 and NLRP3 inflammasomes in vivo and establish a role for these sensors in host resistance to toxoplasmosis. Using mice deficient in IL-18 and IL-18R, we show that the IL-18 cytokine plays a pivotal role by limiting parasite replication to promote murine survival.
INTRODUCTION
The innate immune response plays a critical role in protecting hosts against pathogens. Activation of innate immunity occurs after pattern recognition “sensor” proteins such as the Toll-like receptors (TLRs) or nucleotide-binding domain and leucine-rich repeat-containing (NLR) proteins detect the presence of pathogens, their products, or the danger signals that they induce during active infection (1, 2). Toxoplasma gondii is an intracellular protozoan parasite capable of potently activating innate immunity in the wide range of vertebrate species that it infects (3, 4). In mice, resistance to T. gondii infection is critically dependent on the TLR-associated adaptor protein MyD88, which is required for the induction of protective levels of the proinflammatory cytokines interleukin-12 (IL-12) and gamma interferon (IFN-γ) and the synthesis of nitric oxide (NO) (5–11). The activation and recruitment of inflammatory monocytes to sites of infection are protective, as infection of mice rendered deficient in Gr1+ inflammatory monocytes by antibody depletion results in increased susceptibility to parasite infection (12, 13). Furthermore, chemokine receptor CCR2- and MCP1 (CCL2)-knockout (KO) mice, defective in recruitment of these cells, are also more susceptible (12, 13). Hence, induction of protective immunity against this protozoan pathogen is critically dependent on monocyte and macrophage cell activation.
Macrophages are activated when their cognate receptors detect the presence of microbial products. In the case of cytosolic NLRs, which sense the presence of microbes and/or the damage that their infection induces, activation leads to the assembly of the inflammasome, a multiprotein complex that recruits and activates caspase-1 and/or caspase-11. The murine NLRP3 inflammasome senses a wide range of bacteria, pore-forming toxins, and crystalline danger signals, including alum, amyloid clusters, cholesterol, and asbestos (for a review, see reference 14). In contrast, the murine NLRP1b inflammasome is more restricted; the only characterized activator is the Bacillus anthracis lethal toxin (LT) (15). Either multimeric complex is capable of cleaving the proform of caspase-1, which is typically associated with the rapid death of macrophages, through a process known as pyroptosis (for a review, see references 1 and 2). Pyroptosis, unlike apoptosis, leads to lysis of the cell and release of its intracellular contents. Caspase-1 also cleaves the proinflammatory cytokines IL-1β and IL-18, allowing their secretion from cells (for a review, see references 1 and 2). Whether the inflammasome is activated during Toxoplasma infection, or is capable of altering disease pathogenesis, has thus far been only inferred. An association of polymorphisms in the human Nlrp1 gene with susceptibility to congenital toxoplasmosis was recently reported (16, 17). T. gondii production of cleaved IL-1β in human monocytes is dependent on both caspase-1 and the NLRP3 adaptor protein ASC (18). P2X(7) receptors, which are important in ATP-mediated activation of the NLRP3 inflammasome, have also been shown to influence parasite proliferation in human and murine cells (19). IL-18, a key substrate of inflammasome-activated caspase-1, is known to enhance production of IFN-γ (20), which is a central regulator of Toxoplasma pathogenesis. Furthermore, in vivo administration of IL-1β protects mice from lethal challenge with Toxoplasma (21) and injection of antibodies against the IL-1 receptor (IL-1R) significantly attenuates the protective effect that exogenous IL-12 confers on infected SCID mice (22). Thus, we hypothesized that inflammasome activation might be an important factor mediating murine host resistance to Toxoplasma infection.
In this study, we show that murine macrophages are not susceptible to T. gondii-induced rapid pyroptosis but that NLRP3 inflammasome activation in these cells results in rapid IL-1β cleavage and release. We establish that both NLRP3 and NLRP1 are important in vivo regulators of parasite proliferation and that IL-18 signaling is required to mediate host resistance to acute toxoplasmosis. Our findings establish a role for two inflammasomes in the control of Toxoplasma infection.
RESULTS
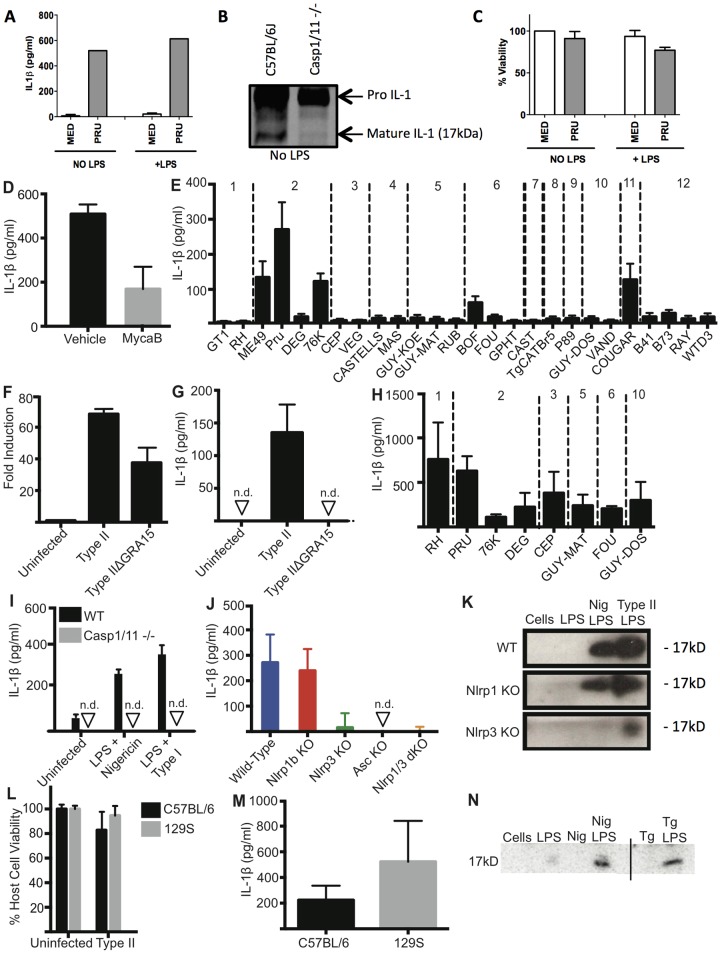
Toxoplasma activates the inflammasome in murine macrophages without inducing cell death.
Induction of protective immunity capable of controlling murine Toxoplasma infection is critically dependent on myeloid cell activation (3). The ability of this parasite to promote caspase-1 activation and the secretion of active IL-1β has recently been established in human and rat monocytes and macrophages (17, 18, 66). To determine if Toxoplasma activates the inflammasome in murine macrophages, we infected unstimulated and lipopolysaccharide (LPS)-primed bone marrow-derived macrophages (BMDMs) prepared from C57BL/6J mice with type II (Pru) parasites and measured IL-1β secretion 24 h after infection (Fig. 1A). Uninfected BMDMs did not produce measurable levels of IL-1β (Fig. 1A), whereas IL-1β was readily detected after infection with type II Toxoplasma regardless of whether the BMDMs were LPS primed or not (Fig. 1A). Western blotting of infected BMDM lysates showed the presence of mature IL-1β and showed that cleavage was dependent on caspase-1/11, since infected BMDMs from caspase-1/11-deficient mice did not possess detectable levels of cleaved IL-1β (Fig. 1B). The detection of mature IL-1β in the Toxoplasma-infected BMDMs indicated inflammasome activation, but the cells did not undergo pyroptosis over 24 h (Fig. 1C). These data support an inflammasome-mediated processing and release of mature IL-1β in the absence of pyroptosis, which have been demonstrated to occur previously (23). Interestingly, IL-18 upregulation and cleavage were not observed in Toxoplasma-infected BMDM or splenocyte lysates over a range of multiplicities of infection (MOIs) and times, and the cytokine was not released from in vitro-infected macrophages or splenocytes (data not shown).
FIG 1 .
Toxoplasma activates the inflammasome in C57BL/6 and 129S BMDMs. BMDMs were primed with 100 ng/ml LPS or left unstimulated for 2 h and subsequently infected with type II parasites (Pru; average MOI, 1) for 24 h. (A) Quantification of IL-1β in supernatants was performed using ELISA. (B) IL-1β cleavage was monitored by Western blotting of cell lysates from C57BL/6NTac or caspase-1/11−/− BMDMs that were infected with type II parasites (Pru; MOI, 0.8) for 24 h. The positions of both pro-IL-1β (37 kDa) and cleaved IL-1β (17 kDa) are indicated. (C) Cell viability of infected cells in panel A was determined at different time points using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. Panels A and C are averages of three experiments. Error bars, ±standard deviations. (D) BMDMs were primed with LPS for 2 h and infected for 24 h with type I parasites (RH) that were pretreated with dimethyl sulfoxide vehicle or mycalolide B (3 µM) for 20 min. IL-1β was measured using ELISA. Data are averages of 2 experiments. Error bars, +standard deviations. (E) C57BL/6 BMDMs were infected with the indicated strains for 24 h. IL-1β was measured using ELISA. Data are the averages of at least 3 experiments per strain. The haplogroup to which the strain belongs is indicated above. Error bars, +standard deviations. (F) C57BL/6 BMDMs were infected for 18 h with Pru (type II) or PruΔGRA15 (MOI, 4) for 18 h, and microarrays were used to determine the fold change in IL-1β mRNA expression levels compared to uninfected macrophages. (G) BMDMs were infected with Pru (type II) or PruΔGRA15 for 24 h. IL-1β was measured using ELISA. Data are representative of 3 experiments. Error bars, +standard deviations. (H) BMDMs were primed for 2 h with LPS and then infected with indicated strains for 24 h (MOI, 4). The haplogroup to which the strain belongs is indicated above. Data are the averages of 3 experiments. Error bars, +standard deviations. (I) IL-1β secretion from primed immortalized murine WT and caspase-1/11−/− macrophages, infected for 24 h with RH. Data shown are from an experiment representative of three. Error bars, +standard deviations. (J) IL-1β secretion from BMDMs prepared from wild-type C57BL/6 mice (blue) or C57BL/6 mice lacking Nlrp1b (red), Nlrp3 (green), Nlrp1b and Nlrp3 (orange), or Asc (yellow), primed for 4 h, and then infected with type I (RH; average MOI, 1) for 24 h. Cytokine secretion below the detection level is indicated on the graph with arrowheads and labeled not detected (n.d.). Data are averages of four experiments. Error bars, +standard deviations. (K) BMDMs described for panel J were primed for 3 h with LPS and then infected with type II parasites (MOI, 1.5) for 24 h. Western blot analysis on concentrated supernatants (25-fold), probing for cleaved IL-1β (17 kDa). (L) Host cell viability in panel L was measured using the MTS assay. Error bars, +standard deviations. (M) BMDMs from C57BL/6 and 129S mice were primed with 100 ng/ml LPS for 2 h and subsequently infected with type II parasites (Pru; average MOI, 0.7) for 24 h. Quantification of IL-1β in supernatants was performed using ELISA. Panels L and M are the averages of 3 experiments. (N) 129S BMDMs were primed for 2 h and infected with type II parasites (MOI, 1.6) for 24 h. Western blot analysis on concentrated supernatants (25-fold), probing for cleaved IL-1β (17 kDa). Nig, nigericin; Tg, T. gondii.
Because IL-1β secretion was consistently dependent on MOI (data not shown), we tested whether parasite invasion was required for IL-1β secretion. Parasites pretreated with mycalolide B, an actin-depolymerizing agent that blocks invasion but allows for secretion of microneme and rhoptry contents, attached but induced significantly smaller amounts of IL-1β secretion (Fig. 1D), indicating that macrophage inflammasome activation was invasion dependent. The small amount of IL-1β secretion by BMDMs infected by mycalolide B-treated parasites was likely due to incomplete inhibition of invasion, as immunofluorescence microscopy performed on the same batch of treated parasites indicated that a small number had still invaded the BMDMs, as evidenced by their intracellular replication (data not shown).
IL-1β secretion correlates with strain differences in NF-κB activation.
Mouse strains differ in their susceptibility to Toxoplasma depending on the infecting strain genotype; haplogroup 2 and 12 (HG2 and HG12) strains are relatively avirulent and readily establish chronic infections, whereas HG1 and HG4 to HG10 strains are acutely virulent. We sought to determine whether Toxoplasma strains differentially activate the murine macrophage inflammasome, or whether secretion of IL-1β correlated with parasite genotype and/or pathogenesis. We infected unprimed BMDMs from C57BL/6J mice with Toxoplasma tachyzoites from all 12 haplogroups for 24 h, a time point at which parasite-induced cell lysis was minimal. Cougar (HG11) and the type II strains, with the exception of DEG, induced IL-1β secretion in unstimulated BMDMs (Fig. 1E). Inflammasome activation is often divided into a signal 1, which is the signal that leads to transcription of Il-1β, and signal 2, which is the signal that leads to the actual activation of caspase-1. Type II, but not type I or III, parasites directly activate the NF-κB transcription factor in both human and murine cells, thereby potentially providing signal 1 for the induction of Il-1β transcription. The secreted dense granule protein GRA15 determines this strain difference in NF-κB activation (24). Indeed, in murine BMDMs, type II IL-1β mRNA induction was partially dependent on type II GRA15 expression (Fig. 1F), while IL-1β secretion of unstimulated BMDMs was completely dependent on GRA15 (Fig. 1G). To determine if non-type II strains can provide signal 2, which leads to the activation of caspase-1 and subsequent cleavage and secretion of IL-1β, we prestimulated BMDMs with LPS for 2 h and subsequently infected them with different Toxoplasma strains. IL-1β was now detected in the medium with no truly apparent differences between strains (Fig. 1H). The IL-1β secreted into the medium also contained the cleaved active IL-1β (17 kDa) as determined by Western blot analysis (see Fig. S2 in the supplemental material).
Toxoplasma activation of the murine inflammasome in BMDMs is dependent on caspase-1/11 and NLRP3.
To determine the components necessary for IL-1β secretion, we infected immortalized macrophages that lacked caspase-1 and -11 and showed that IL-1β secretion was completely eliminated, as expected (Fig. 1I). To determine the inflammasome components necessary for IL-1β secretion, we infected BMDMs from C57BL/6 mice that lacked Nlrp1b, Nlrp3, or both Nlrp1b and Nlrp3, or the inflammasome adaptor ASC. We found IL-1β secretion by primed BMDMs upon Toxoplasma type I infection to be mostly dependent on ASC and the NLRP3 inflammasome (Fig. 1J). Similar results were obtained after type II infection (see Fig. S3 in the supplemental material). The greatly reduced amount of cleaved active IL-1β in the supernatant of Nlrp3-deficient Toxoplasma-infected BMDMs compared to the amount present in the supernatant of wild-type (WT) or Nlrp1b-deficient infected BMDMs confirmed the importance of the NLRP3 inflammasome in Toxoplasma-mediated inflammasome activation in vitro (Fig. 1K). Thus, Toxoplasma induction of IL-1β secretion by murine BMDMs is highly dependent on the NLRP3 inflammasome and requires caspase-1 activation.
Inflammasome-mediated BMDM death and cytokine processing are independent of Nlrp1a and Nlrp1b alleles.
Despite the activation of caspase-1 in Toxoplasma-infected cells, we did not observe any macrophage pyroptosis. Previous reports have shown that five polymorphic Nlrp1b alleles exist among inbred mice which control sensitivity to anthrax LT-induced pyroptosis (15). To test if the >100-amino-acid (aa) differences in Nlrp1b between C57BL/6J and 129S mice were the basis for resistance to parasite-induced pyroptosis, we compared BMDMs from the two strains. We observed no difference in cell viability between the C57BL/6J and 129S BMDMs (Fig. 1L). Thus, consistent with a major role for NLRP3 inflammasome activation, BMDMs from either 129S or C57BL/6 mice produced active IL-1β (Fig. 1M and N) without associated pyroptosis upon type I or II Toxoplasma strain infection. Furthermore, the fact that 129S BMDMs do not express the highly conserved NLRP1a protein (25) and are caspase-11 deficient (26) but present a robust IL-1β response also eliminated a role for NLRP1a and caspase-11 in cytokine maturation induced by Toxoplasma.
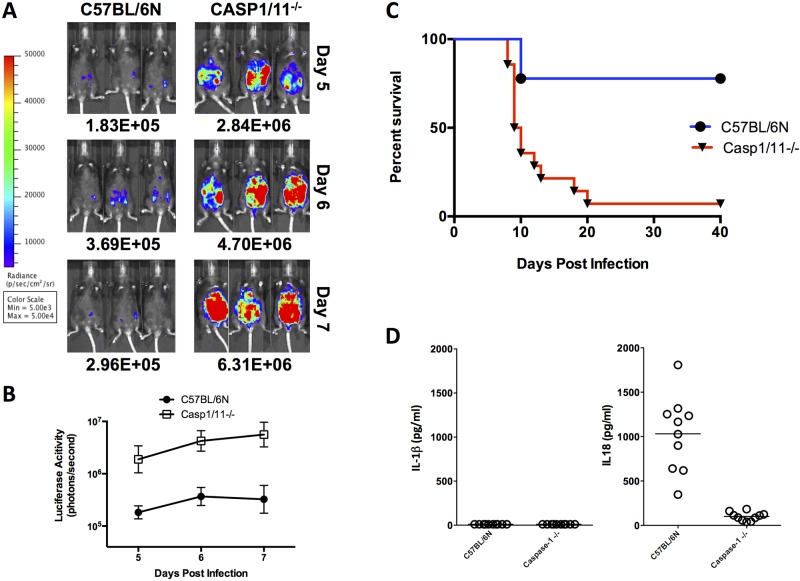
Murine resistance to Toxoplasma infection is controlled by caspase-1/11-dependent inflammasome activation.
Whether inflammasome activation is important to murine resistance to infection in vivo has not yet been established. We infected mice deleted for the caspase-1/11 genes with 10,000 type II 76K green fluorescent protein-luciferase (GFP-LUC) tachyzoites intraperitoneally (i.p.) and tested for susceptibility to acute infection by monitoring mean survival time (MST), parasite growth, dissemination, and the production of IL-1β and IL-18. In the absence of caspase-1/11 proteins, mice had a 10- to 20-fold-higher parasite load (Fig. 2A and B) and were highly susceptible to acute infection (Fig. 2C). In contrast, the majority of C57/BL6NTac control mice survived acute infection and established chronic infections (Fig. 2C). Surprisingly, serum levels of systemic IL-1β never exceeded 10 pg/ml on day 5 (Fig. 2D, graph on left) or day 9 (data not shown) for either mouse strain. IL-18 levels, however, were significantly higher following infection, ranging from 0.5 to 2.0 ng/ml in C57BL/6NTac mice on day 5 (Fig. 2D), to strikingly high levels exceeding 10 ng/ml by day 9, compared to <200 pg/ml in caspase-1/11-deficient mice (Fig. 2D) or uninfected controls (data not shown).
FIG 2 .
Parasite load, survival, and systemic IL-18 levels in caspase-1/11-deficient mice. (A) Bioluminescence imaging (BLI) of infected caspase-1/11 knockouts and controls on days 5 to 7 following infection with 76K GFP-LUC (10,000 tachyzoites i.p.). Images shown are for 3 mice. (B) Quantifications are from 8 mice imaged/group. (C) Aggregate survival of caspase-1/11-knockout mice (n = 13/group) compared with WT C57BL/6NTac control mice (n = 10/group). (D) IL-18 measurements in serum of caspase-1/11-deficient mice on day 5 after infection were significantly different from WT (P < 0.001) when infected with 76K GFP-LUC. No detectable levels of IL-1β were detected in circulation.
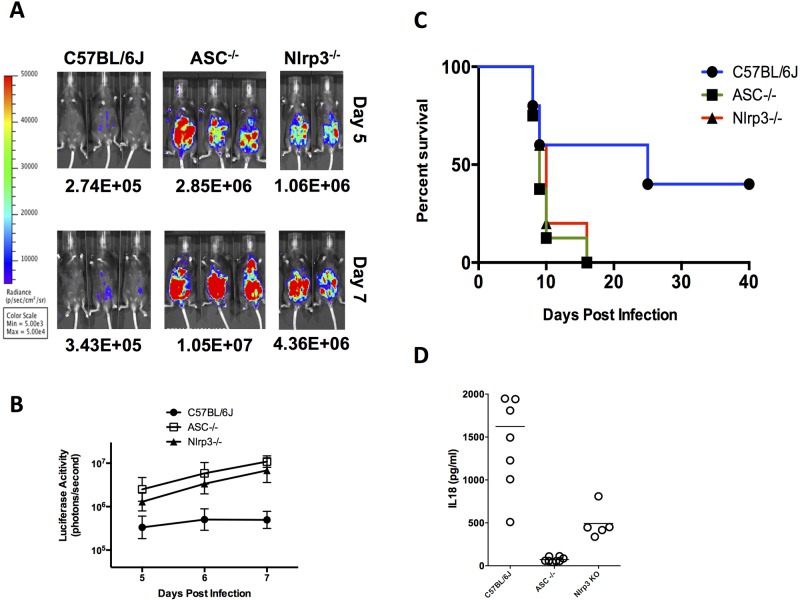
ASC and NLRP3 inflammasome activation controls Toxoplasma proliferation and host resistance.
We next investigated the role of ASC in murine susceptibility to Toxoplasma infection, since this adaptor protein is required to mediate the activation of multiple inflammasomes (27). If inflammasome activation controls Toxoplasma resistance, we hypothesized that mice rendered deficient in this protein will be more susceptible to acute infection. Asc-deficient mice consistently had ~20-fold or greater parasite loads at days 5 to 7 postinfection (Fig. 3A and B) and generally succumbed to infection by days 8 to 10 (Fig. 3C), in contrast to wild-type control mice, the majority of which survived acute infection at day 20 (Fig. 3C). The Asc-deficient mice likewise failed to induce detectable levels of systemic IL-18 (Fig. 3D) or IL-1β (data not shown) during acute infection.
FIG 3 .
Parasite load, survival, and systemic IL-18 levels in ASC- and NLRP3-knockout mice following Toxoplasma challenge. (A) Bioluminescence imaging of 76K GFP-LUC-infected mice (various strains, 10,000 tachyzoites, i.p. route) on days 5 to 7 following infection is shown. Images shown are from two or three representative mice from 2 to 6 mice/group from one representative experiment. The experiment shown is one of 3 (WT) or 2 (ASC and NLRP3) independent experiments. (B) P values (t test) comparing luciferase activity for each knockout strain to C57BL/6J are <0.05. (C) Aggregate survival curve of ASC (n = 8)- and NLRP3 (n = 5)-knockout mice compared with WT C57BL/6J control mice (n = 13). (D) IL-18 measurements in serum of deficient mice on day 5 after infection. No detectable levels of IL-1β were detected in circulation.
To determine whether the NLRP3 inflammasome sensor is sufficient to confer this murine resistance to the Toxoplasma phenotype, Nlrp3−/− mice on the C57BL/6J background were infected intraperitoneally with 10,000 76K GFP-LUC tachyzoites. Nlrp3−/− mice were more susceptible than their wild-type (WT) controls and possessed 10-fold or higher parasite burdens at days 5 to 7, and the majority of mice died by day 10 postinfection (Fig. 3C). These infected mice produced intermediate levels of systemic IL-18, significantly greater than those of the Asc-deficient mice but not equivalent to those of the WT (Fig. 3D). They did not produce measurable levels of IL-1β (data not shown), similar to what was observed with all infections of WT mice (Fig. 2D and data not shown). Infection with another type II strain (Pru GFP-LUC) produced similar results (data not shown), indicating that the phenotype was not attributable to an anomalous Toxoplasma clone-specific effect or the genome integration site of the GFP-LUC gene, as both type II strains activated the inflammasome in vivo. These results indicate that murine resistance to acute infection with Toxoplasma is highly dependent on the activation of the NLRP3 inflammasome.
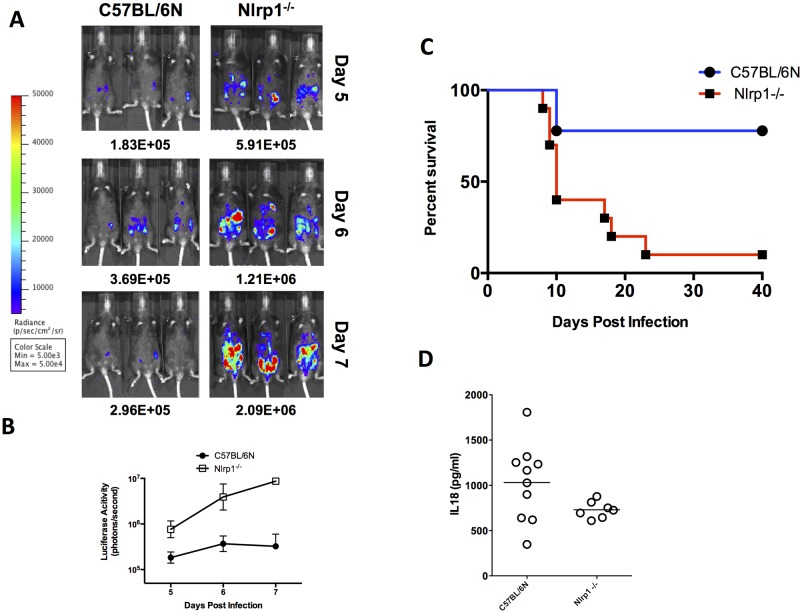
NLRP1 inflammasome activation also controls Toxoplasma proliferation and host resistance.
Because the infected Nlrp3-deficient mice still produced IL-18 at levels higher than those of Asc-deficient mice, we hypothesized that more than one inflammasome is activated in vivo to produce IL-18 during Toxoplasma infection. To test this prediction, we infected mice deficient at the Nlrp1 locus encompassing Nlrp1abc with 10,000 76K GFP-LUC tachyzoites to determine if NLRP1 activation contributes to murine resistance during Toxoplasma infection. Nlrp1abc-deficient mice had only 3- to 5-fold-higher parasite loads than did WT mice across days 5 to 7 (Fig. 4B). Nlrp1abc−/− mice also died acutely, succumbing to infection between days 16 and 22 (Fig. 4C). The delayed MST kinetics was consistent with the decreased parasite burden in comparison to the Nlrp3-deficient mice. Nlrp1abc-deficient mice produced intermediate levels of systemic IL-18, significantly greater than those of the Asc-deficient mice but not equivalent to those of the WT (Fig. 4D). No measurable levels of circulating IL-1βwere found on day 5 or 9 after infection in these mice or their WT controls (data not shown).
FIG 4 .
Parasite load, survival, and systemic IL-18 levels in NLRP1-knockout mice following Toxoplasma challenge. (A) Bioluminescence imaging of 76K GFP-LUC-infected mice (various strains, 10,000 tachyzoites, i.p. route) on days 5 to 7 following infection is shown. Images shown are three representative mice from 4 to 6 mice/group from one representative experiment. The experiment shown is one of 2 WT or 2 NLRP1 independent experiments. (B) P values (t test) comparing luciferase activity for each knockout strain to C57BL/6NTac are <0.05. (C) Aggregate survival curve of NLRP1 (n = 10)-knockout mice compared with WT C57BL/6NTac control mice (n = 10). (D) IL-18 measurements in serum of deficient mice on day 5 after infection. No detectable levels of IL-1β were detected in circulation.
Mice deficient in signaling and secretion of IL-18 are highly susceptible to Toxoplasma infection.
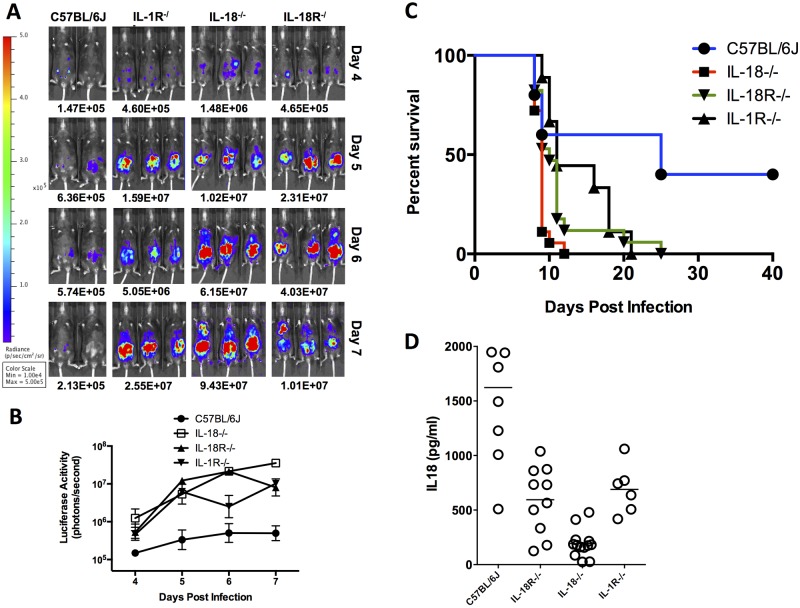
Although no systemic IL-1β was detectable in vivo, infection of NLRP3-deficient BMDMs showed markedly diminished levels of IL-1β (Fig. 1). To test if IL-1β possesses a biologically important role locally and is capable of influencing murine resistance, we infected IL-1R−/− mice with 10,000 76K GFP-LUC tachyzoites. All IL-1R-deficient mice succumbed to infection but with a delayed kinetics compared to ASC-, caspase-1/11-, and Nlrp3-deficient mice (Fig. 5C). These mice supported higher parasite loads and had intermediate levels of IL-18 in their serum compared to WT C57BL6/J mice (Fig. 5A, B, and D), indicating that despite the absence of measurable IL-1β in circulation, IL-1 signaling does play a contributing role in the protection against murine toxoplasmosis.
FIG 5 .
Parasite load, survival, and systemic IL-18 levels in IL-18- and IL-18R-knockout mice. (A) Bioluminescence imaging of 76K GFP-LUC-infected mice (various strains, 10,000 tachyzoites, i.p. route) on days 4 to 7 following infection is shown. Images shown are 2 to 3 representative mice from 3 to 6 mice/group. The experiment shown is one of 2 IL-1β, 4 IL-18, or 4 IL-18R independent experiments. (B) P values (t test) comparing luciferase activity for each knockout strain to C57BL/6J are <0.05. (C) Aggregate survival curve of IL-1R (n = 9)-, IL-18 (n = 19)-, and IL-18R (n = 13)-knockout mice compared with WT C57BL/6J control mice (n = 13). (D) IL-18 measurements in serum of deficient mice on day 5 after infection. No detectable levels of IL-1β were detected in circulation.
Intriguingly, the level of IL-18 in the circulation of infected WT mice correlated with decreased parasite burden and increased survival. We hypothesized that inflammasome activation and the production of high systemic IL-18 might play an important role in the relative resistance of WT mice. To test our hypothesis, we infected IL-18−/− and IL-18R−/− mice. Both types of mice were highly susceptible to acute Toxoplasma infection and consistently had 20- to >100-fold-increased parasite loads at days 5 to 7 postinfection, greater than that found in the Asc−/− mice (Fig. 5A and B). These mice typically succumbed to infection by day 8 or 9 (Fig. 5C), suggesting that the presence of IL-18 is protective in C57BL/6 mice. These data indicate that the production and secretion of activated IL-18 are associated with controlling parasite proliferation and murine resistance to acute toxoplasmosis.
DISCUSSION
Generation of a robust innate immune response is required to orchestrate murine resistance against the intracellular pathogen T. gondii, as well as a wide spectrum of other pathogenic agents (for a review, see reference 3). Resistance to Toxoplasma infection is critically dependent on the TLR-associated adaptor protein MyD88 and induction of IL-12, IFN-γ, and the synthesis of nitric oxide (NO). In this study, we show that in vivo generation of host protective immunity against Toxoplasma is also highly dependent on the inflammasome sensors NLRP1 and NLRP3 and the secretion of the caspase-1-dependent proinflammatory cytokine IL-18. Infection of mice deficient in NLRP3, NLRP1abc, caspase-1/11, or the inflammasome adaptor protein ASC led to decreased levels of circulating IL-18, increased parasite replication, and death. Using mice deficient in IL-18 and IL-18R, we show that this cytokine plays an important role in limiting parasite replication to promote murine survival.
IL-18, like IL-1β, has been extensively linked to both protective immune responses and disease induction. IL-18 mediates enhancement of innate resistance to acute toxoplasmosis by triggering IFN-γ induction in immune cells, especially T and NK cells, and works in synergy with IL-12. It has previously been used as a protective treatment (28, 29), and IL-18 depletion by antibodies significantly alters murine susceptibility upon infection with lethal doses of Toxoplasma (30). In fact, IL-18 was at one time known as “IFN-γ-inducing factor” (31). The inactive pro-IL-18 form is constitutively expressed in a wide range of cell types (32) but requires processing by caspase-1 to promote its secretion, as evidenced by the drastically depleted levels of IL-18 during infection of caspase-1/11−/− mice (Fig. 2D), and promote the induction of IFN-γ production in vivo (33, 34). Interestingly, Asc-deficient mice produced even less circulating IL-18, indicating that other factors such as caspase-8 (35) may contribute to IL-18 processing.
The role of IFN-γ in resistance to Toxoplasma is extensively documented (for a review, see references 3 and 4). IFN-γ activates cellular pathways that promote resistance to toxoplasmosis through multiple mechanisms, including activation of the interferon-inducible GTPases (IRG-GTPases) (36, 37) and NO regulation (9), processes certainly dependent on induction of IFN-γ by IL-18 produced following NLR inflammasome activation. However, IL-18 has also been linked to pathology during infection with type I strains of Toxoplasma, and IL-18 depletion resulted in enhanced survival by limiting the propathologic immune response that these virulent strains induce (30, 38). Hence, the balance between the protective and pathological roles of IL-18 is likely highly dependent on mouse genetics, Toxoplasma strain differences, challenge doses, routes of infection, and rates of disease progression. Previous work using a low-dose type II (PTG) infection in caspase-1-deficient mice (now known to be caspase-1/11 deficient) concluded that these mice were not altered in Toxoplasma susceptibility relative to wild-type control mice (39). However, this study was performed with a parasite dose that does not typically induce measurable levels of systemic IL-18 (30), and the mice used had a mixed 129/B6 background, which itself may influence pathogenesis. Our results, using mice sufficiently backcrossed onto a C57BL/6NTac background and a parasite inoculum that induces systemic IL-18, show a role for caspase-1 and IL-18 in murine resistance. IL-18 concentrations are known to vary through the course of infection and are clearly dependent on the parasite strain and inoculum used (30, 38). Our results suggest that this cytokine plays a pivotal role in mediating acute toxoplasmosis, with the cytokine playing an important early role in the control of parasite replication (Fig. 5B). How exactly IL-18 mediates this protection requires further studies. Later in infection, however, high levels of IL-18 have previously been shown to cause dysregulated induction of propathologic cytokine levels that contribute to lethality in high-dose, virulent infections (30, 38).
The recruitment of inflammatory monocytes to sites of infection is essential to control parasite growth and dissemination in murine models of toxoplasmosis (12, 13). In rats, control of parasite proliferation and dissemination in vivo is controlled by the Toxo1 locus (40). Our recent work showed that macrophages from Toxoplasma-resistant rat strains (e.g., LEW and SHR) undergo pyroptosis in response to inflammasome activation induced by parasite infection, and this rapid cell death is sufficient to limit parasite replication and promote sterile cure (66). In previous work by Miao et al., caspase-1-induced pyroptotic cell death was also identified as an innate immune mechanism to protect against intracellular pathogen infection (41). In their study, the authors used a panel of mouse strains deficient in IL-1β, IL-18, IL-1βR, or various combinations of those to show a dispensable role for IL-1β and IL-18 in the clearance of Salmonella enterica serovar Typhimurium that expresses flagellin, suggesting that innate control of bacterial infection is occurring by pyroptosis, without a requirement to induce an overt inflammatory response. In this study, we show that in vitro Toxoplasma infection of murine bone marrow-derived macrophages primarily activates the NLRP3 inflammasome, resulting in the rapid production and cleavage of IL-1β, but does not induce pyroptosis. Interestingly, although Toxoplasma-infected macrophages showed efficient caspase-1/11-dependent IL-1β cleavage and secretion, these cells did not upregulate, cleave, or secrete their preexisting pools of IL-18. Furthermore, splenocytes also did not show any IL-18 cleavage following infection. Paradoxically, significant concentrations of IL-1β were not detected following infection in any mouse strain, whereas high levels of IL-18 were found in serum in all infection studies that we performed with different Toxoplasma strains. Because activation of the murine inflammasome does not affect Toxoplasma growth in macrophages (data not shown) and does not induce pyroptosis, our results suggest that IL-18 activation and not pyroptosis is the genetic basis for in vivo inflammasome-mediated control of parasite proliferation. Although the in vivo cellular source for this inflammasome-generated IL-18 is not known, it is likely of nonmyeloid origin, and bone marrow chimera studies should be performed to address this question. Our results also suggest that NLRP1-mediated events may be more important in vivo and that activation of NLRP1 may likewise occur in cells other than macrophages.
The role of inflammasome activation in the pathogenesis of Toxoplasma infection in human infection has recently been suggested (16, 17). Polymorphisms in the human NLRP1 gene are associated with susceptibility to congenital toxoplasmosis, and NLRP1 contributes to controlling parasite growth in human monocytes. A recent study in human macrophages provides compelling evidence that the inflammasome components ASC and caspase-1 regulate the release of IL-1β and that the type II allele of the parasite dense granule protein GRA15, which activates NF-κB nuclear translocation, is necessary for maximal induction of this cytokine (18). Indeed, our in vitro infection data show that Toxoplasma murine inflammasome-mediated secretion of IL-1β is strain dependent and that only parasites expressing the GRA15 type II allele, which directly activates NF-κB, were able to induce secretion of IL-1β in unprimed BMDMs (Fig. 1). While the relative and contributing roles of IL-1β compared to IL-18 remain to be determined in the control of acute toxoplasmosis, our preliminary studies using IL-1 receptor-knockout mice on a mixed background argue that IL-1β plays a less significant role in the control of parasite infection than it does in IL-18 or IL-18R knockouts (Fig. 5) In vivo administration of IL-1β in LPS-primed caspase-1/11-deficient mice has previously been shown to increase IL-6 (34), so it is conceivable that IL-1β functions locally to induce increased levels of IL-6 capable of altering inflammation-induced changes in myeloid output that impact Toxoplasma pathogenesis (42).
Of the two NLR inflammasomes activated, we found that murine resistance to acute infection was principally dependent on activation of the NLRP3 receptor both in vitro (Fig. 1J) and in vivo (Fig. 3). Several reports have linked P2X(7) receptor, a potent activator of the NLRP3 inflammasome, with control of acute toxoplasmosis (16, 19, 43, 44). How and in what cell type Toxoplasma activates the murine NLRP3 inflammasome or why its activation does not lead to rapid macrophage death or IL-18 processing is enigmatic. Regulators of the NLRP3 inflammasome include ATP, the guanylate-binding protein 5 (GBP5), cellular stresses that alter calcium and potassium concentrations, redox status, and the unfolded protein response (UPR) (for a review, see reference 45). Importantly, Toxoplasma encodes a variety of virulence effector proteins that specifically inactivate the host endoplasmic reticulum (ER)-bound transcription factor ATF6β and induction of the UPR during ER stress (46), affect the recruitment of 65-kDa guanylate-binding proteins (GBPs) (47, 48), or alter calcium and potassium efflux to signal Toxoplasma egress (49), perhaps indicating that the parasite has specifically evolved effector proteins to minimize NLR inflammasome activation to alter its pathogenesis.
Our work also identified Toxoplasma as the second pathogen, after B. anthracis, whose pathogenesis is altered by expression of the murine NLRP1 inflammasome (15). We show that the Nlrp1 locus is capable of regulating parasite proliferation in vivo, with Nlrp1 knockout mice possessing significantly higher parasite burdens following Toxoplasma infection. Although the majority of NLRP1-deficient mice died acutely (Fig. 4), they were, however, less susceptible to infection than were caspase-1/11-, Asc-, Nlrp3-, IL-18-, or IL-18R-deficient mice in the same genetic background and possessed only 5- to 10-fold-higher parasite loads than in WT infections. How Toxoplasma activates the NLRP1 inflammasome is unclear. Activation of rodent NLRP1 inflammasomes by the B. anthracis lethal toxin (LT) occurs via proteolytic cleavage at a specific consensus sequence in the polymorphic N terminus of NLRP1 (50, 51). In human infection, NLRP1 polymorphism variants are likewise known to alter the susceptibility to congenital toxoplasmosis (17). One logical hypothesis is that the Toxoplasma-encoded effector molecule responsible for activation of NLRP1 is, like LT, a protease. Toxoplasma secretes a wide range of proteases (52–57), and similar induction of IL-1β observed upon infection of primed BMDMs with any Toxoplasma strain suggests that the putative protease, or factor responsible for activation of NLRP1, is not likely to be Toxoplasma strain specific or is at least conserved among the majority of strains. Alternatively, polymorphisms in Nlrp1 could affect the interaction with a different host “sensor” protein that serves as the adaptor for assembly and activation of the NLRP1 inflammasome, as has been previously described for the NLRC4/NAIP5/NAIP6 inflammasome recognition of flagellin (58, 59).
In summary, we establish that both NLRP3 and NLRP1 are important in vivo regulators of Toxoplasma proliferation and that IL-18 signaling is required to mediate host resistance to acute toxoplasmosis. Our findings also indicate that innate resistance to acute toxoplasmosis is dependent on the activation of both TLR and NLR sensors that cooperate to detect the presence of pathogen products or the danger signals that they induce during active infection. The identification of the Toxoplasma factor that mediates NLR inflammasome activation may contribute new insight into the development of therapeutic options to combat this important human pathogen.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in strict accordance with guidelines from the NIH and the Animal Welfare Act, under protocols approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (protocols LPD-8E and LPD-22E), and the MIT Committee on Animal 441 Care (assurance number A-3125-01).
Material.
Ultrapure LPS was purchased from Calbiochem/EMD Biosciences (San Diego, CA). Luciferin was purchased from Caliper Life Sciences (Hopkinton, MA). Nigericin was purchased from Invivogen (San Diego, CA). Mycalolide B was purchased from Wako (Richmond, VA).
Mice and NLRP1 expression status-based nomenclature.
IL-18- and IL-18 receptor (IL-18R)-knockout mice on the C57BL/6J background (>10 backcrosses) and IL-1R-deficient mice on a partially backcrossed 129 × C57BL/6J background were obtained from Jackson Laboratories (Bar Harbor, ME). Caspase-1-knockout mice have been previously described (60) and were backcrossed to C57BL/6NTac mice for 10 generations. These caspase-1-knockout mice are also deficient in caspase-11 (26). Mice deleted for all three Nlrp1 genes in the murine Nlrp1abc locus (C57BL/NTac background), as well as those deleted only for Nlrp1b (C57BL/6J background), have been previously described (61, 62). Mice deleted at Nlrp3 (C57BL/6J background) (63) and Asc (C57BL/6J background) (60) have been previously described.
Parasites.
Tachyzoites from luciferase-expressing type I (RH) and type II (76K or Prugniaud) T. gondii parasites were used for all studies. The following strains (haplogroup/type in parentheses) were used in a survey of effects on murine BMDMs: GT1 (I), ME49 (II), DEG (II), CEP (III), VEG (III), CASTELLS (IV), MAS (IV), GUY-KOE (V), GUY-MAT (V), RUB (V), BOF (VI), GPHT (VI), CAST (VII), TgCATBr5 (VIII), P89 (IX), GUY-DOS (X), VAND (X), Cougar (XI), B41 (XII), B73 (XII), RAY (XII), and WTD3 (XII). The generation of luciferase-expressing parasites using the plasmid pDHFR-Luc-GFP gene cassette has been described previously (64). To construct the RH, Prugniaud, and 76K GFP-LUC strains, pDHFR-Luc-GFP was linearized with NotI, parasites were electroporated, and those with stable GFP expression were isolated by fluorescence-activated cell sorting and cloned by limiting dilution. Generation of Pru GRA15-knockout (KO) parasites has been previously described (24). All parasite strains were routinely passaged in vitro in monolayers of human foreskin fibroblasts (HFFs) at 37°C in the presence of 5% CO2 and quantified by hemocytometer counts prior to infection studies. In some experiments, mycalolide B (3 µM, 20 min) was used to pretreat isolated parasites prior to washing in phosphate-buffered saline (PBS) (3×) before infections.
Cell culture.
Bone marrow-derived macrophages (BMDMs) were cultured in complete Dulbecco’s modified Eagle’s medium (DMEM) with 20% L929 cell culture supernatant for 7 days. L929 mouse fibroblast cells were grown in DMEM supplemented with 10% fetal bovine serum, 10 mM HEPES, and 50 µg/ml gentamicin (all obtained from Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. BMDMs with or without LPS priming (0.1 µg/ml, 2 h) were infected with Toxoplasma at various multiplicities of infection (MOIs), and cell viability was assessed at 24 h using the CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison, WI). Culture supernatants were removed for cytokine measurements by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) or Western blotting, following concentration using Amicon filters (3,000-molecular-weight cutoff) (Millipore, Billerica, MA) or Spin-X UF 500 concentrators (5,000-molecular-weight cutoff) (Corning, United Kingdom). For Western blots, anti-mouse IL-1β (Abcam, Cambridge, MA) or anti-caspase-1 antibody (Abcam, Cambridge, MA) was used as the primary antibody. Secondary antibodies were from Jackson Immunoresearch (West Grove, PA). Immun-Star Western C substrate (Bio-Rad, Hercules, CA) and a charge-coupled device camera (Chemidoc XRS; Bio-Rad) were used for visualization. All immortalized macrophage cell lines (WT and caspase-1/11−/−) were grown in complete DMEM with 10% L929-conditioned medium.
Microarray analysis.
Microarray analyses were performed as previously described (65).
Mouse infections.
Mice (male and female, 8 to 12 weeks old) were infected intraperitoneally (i.p.) with either 10,000 (76K) or 1,200 (Pru) type II tachyzoites diluted in 400 µl of phosphate-buffered saline. Mice were imaged on successive days (typically days 4 to 9) postinfection, and parasite burden was quantified by firefly luciferase activity using an IVIS BLI system from Caliper Life Sciences. Mice were injected i.p. with 3 mg of d-luciferin substrate (prepared in 200 µl of PBS) and imaged for 5 min to detect photons emitted, as previously described (64). Mice were bled by tail vein at day 5 and/or day 9 after infection. Blood collection was performed in either serum collector or Microtainer EDTA tubes (Sarstedt, Newton, NC). IL-1β and IL-18 were measured by ELISA (R&D Systems, Minneapolis, MN).
SUPPLEMENTAL MATERIAL
Type II parasites activate the inflammasome without inducing cell death. BMDMs were primed with 100 ng/ml LPS or left unstimulated for 2 h and subsequently infected with type II parasites (Pru; MOI, 0.4) for 24 h. (A) Quantification of IL-1β in supernatants was performed using ELISA. (B) Cell viability of infected cells in panel A was determined at different time points using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. Download
Type I and type IV parasites induce cleavage of pro-IL-1β. C57BL/6 BMDMs were primed with 100 ng/ml LPS for 2 h and infected with type I (RH) or type IV (MAS) parasites (MOI, 5) for 24 h. Western blot analysis was performed on concentrated supernatant (25-fold), probing for pro-IL-1β (37 kDa) and active IL-1β (17 kDa). Download
Type II parasites activate the Nlrp3 inflammasome. IL-1β secretion from BMDMs prepared from wild-type C57BL/6 mice (blue) or C57BL/6 mice lacking Nlrp1b (red), Nlrp3 (green), or Nlrp1b and Nlrp3 (orange) primed for 4 h and then infected with type II parasites (Pru; MOI, 0.8) for 24 h. The figure represents one experiment. Error bars, +standard deviations. Download
ACKNOWLEDGMENTS
This work was supported in part by the Intramural Research Program of the NIH and NIAID (M.E.G., S.H.L., and A.S.). G.G. was supported by a research fellowship award from the Crohn’s & Colitis Foundation of America (CCFA) and is a CCFA Helmsley Scholar. K.M.C. was supported by NIH grant AI104170. J.P.J.S. was supported by R01-AI080621 and a Pew Scholar in the Biomedical Sciences Award. M.E.G. is a scholar of the Canadian Institute for Advanced Research (CIFAR) Program for Integrated Microbial Biodiversity.
Footnotes
Citation Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, Masters S, Sher A, Leppla SH, Moayeri M, Saeij JPJ, Grigg ME. 2014. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio 5(1):e01117-13. doi:10.1128/mBio.01117-13.
REFERENCES
- 1. Lamkanfi M, Dixit VM. 2012. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28:137–161. 10.1146/annurev-cellbio-101011-155745 [DOI] [PubMed] [Google Scholar]
- 2. Song DH, Lee JO. 2012. Sensing of microbial molecular patterns by Toll-like receptors. Immunol. Rev. 250:216–229. 10.1111/j.1600-065X.2012.01167.x [DOI] [PubMed] [Google Scholar]
- 3. Hunter CA, Sibley LD. 2012. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10:766–778. 10.1038/nrmicro2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melo MB, Jensen KD, Saeij JP. 2011. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 27:487–495. 10.1016/j.pt.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan IA, Schwartzman JD, Matsuura T, Kasper LH. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. U. S. A. 94:13955–13960. 10.1073/pnas.94.25.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LaRosa DF, Stumhofer JS, Gelman AE, Rahman AH, Taylor DK, Hunter CA, Turka LA. 2008. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A. 105:3855–3860. 10.1073/pnas.0706663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997–6001 [DOI] [PubMed] [Google Scholar]
- 8. Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045–4054 [PubMed] [Google Scholar]
- 9. Scharton-Kersten TM, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261–1273. 10.1084/jem.185.7.1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sher A, Collazzo C, Scanga C, Jankovic D, Yap G, Aliberti J. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27:521–528. 10.1385/IR:27:2-3:521 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. 10.1126/science.3128869 [DOI] [PubMed] [Google Scholar]
- 12. Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. 2008. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29:306–317. 10.1016/j.immuni.2008.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robben PM, LaRegina M, Kuziel WA, Sibley LD. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761–1769. 10.1084/jem.20050054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinon F, Mayor A, Tschopp J. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229–265. 10.1146/annurev.immunol.021908.132715 [DOI] [PubMed] [Google Scholar]
- 15. Boyden ED, Dietrich WF. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38:240–244. 10.1038/ng1724 [DOI] [PubMed] [Google Scholar]
- 16. Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, Miller EN, Fuller SJ, Wiley JS, Castellucci L, Boyer K, Peixe RG, Kirisits MJ, Elias LS, Coyne JJ, Correa-Oliveira R, Sautter M, Smith NC, Lees MP, Swisher CN, Heydemann P, Noble AG, Patel D, Bardo D, Burrowes D, McLone D, Roizen N, Withers S, Bahia-Oliveira LM, McLeod R, Blackwell JM. 2010. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 11:374–383. 10.1038/gene.2010.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, Montpetit A, Cavailles P, Bisanz C, Cesbron-Delauw MF, Fournié GJ, McLeod R. 2011. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect. Immun. 79:756–766. 10.1128/IAI.00898-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gov L, Karimzadeh A, Ueno N, Lodoen MB. 2013. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4(4):e00255-13. 10.1128/mBio.00255-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC. 2010. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J. Immunol. 184:7040–7046. 10.4049/jimmunol.1000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Mühl H, Yoon DY, Reznikov LL, Kim SH, Rubinstein M. 1998. Overview of interleukin-18: more than an interferon-gamma inducing factor. J. Leukoc. Biol. 63:658–664 [PubMed] [Google Scholar]
- 21. Chang HR, Grau GE, Pechère JC. 1990. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology 69:33–37 [PMC free article] [PubMed] [Google Scholar]
- 22. Hunter CA, Chizzonite R, Remington JS. 1995. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 155:4347–4354 [PubMed] [Google Scholar]
- 23. Broz P, von Moltek J, Jones JW, Vance RE, Monack DM. 2010. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8:471–483. 10.1016/j.chom.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208:195–212. 10.1084/jem.20100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sastalla I, Crown D, Masters SL, McKenzie A, Leppla SH, Moayeri M. 2013. Transcriptional analysis of the three Nlrp1 paralogs in mice. BMC Genomics 14:188. 10.1186/1471-2164-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- 27. Latz E, Xiao TS, Stutz A. 2013. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13:397–411. 10.1038/nri3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai G, Kastelein R, Hunter CA. 2000. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect. Immun. 68:6932–6938. 10.1128/IAI.68.12.6932-6938.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yap GS, Ortmann R, Shevach E, Sher A. 2001. A heritable defect in IL-12 signaling in B10.Q/J mice. II. Effect on acute resistance to Toxoplasma gondii and rescue by IL-18 treatment. J. Immunol. 166:5720–5725 [DOI] [PubMed] [Google Scholar]
- 30. Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 167:4574–4584 [DOI] [PubMed] [Google Scholar]
- 31. Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88–91. 10.1038/378088a0 [DOI] [PubMed] [Google Scholar]
- 32. Puren AJ, Fantuzzi G, Dinarello CA. 1999. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA 96:2256–2261. 10.1073/pnas.96.5.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619–623. 10.1038/386619a0 [DOI] [PubMed] [Google Scholar]
- 34. Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206–209. 10.1126/science.275.5297.206 [DOI] [PubMed] [Google Scholar]
- 35. Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, Fitzgerald KA, Marshak-Rothstein A, Latz E. 2012. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 189:5508–5512. 10.4049/jimmunol.1202121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Howard JC, Hunn JP, Steinfeldt T. 2011. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr. Opin. Microbiol. 14:414–421. 10.1016/j.mib.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 37. Hunn JP, Feng CG, Sher A, Howard JC. 2011. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mamm. Genome 22:43–54. 10.1007/s00335-010-9293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gavrilescu LC, Denkers EY. 2001. IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 167:902–909 [DOI] [PubMed] [Google Scholar]
- 39. Hitziger N, Dellacasa I, Albiger B, Barragan A. 2005. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell. Microbiol. 7:837–848. 10.1111/j.1462-5822.2005.00517.x [DOI] [PubMed] [Google Scholar]
- 40. Cavaillès P, Sergent V, Bisanz C, Papapietro O, Colacios C. M, Subra JF, Lagrange D, Calise M, Appolinaire S, Faraut T, Druet P, Saoudi A, Bessieres MH, Pipy B, Cesbron-Delauw MF, Fournié GJ. 2006. The rat Toxo1 locus directs toxoplasmosis outcome and controls parasite proliferation and spreading by macrophage-dependent mechanisms. Proc. Natl. Acad. Sci. U. S. A. 103:744–749. 10.1073/pnas.0506643103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11:1136–1142. 10.1038/ni.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chou DB, Sworder B, Bouladoux N, Roy CN, Uchida AM, Grigg M, Robey PG, Belkaid Y. 2012. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during Toxoplasma gondii infection. J. Leukoc. Biol. 92:123–131. 10.1189/jlb.1011527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corrêa G, Marques da SC, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. 2010. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 12:497–504. 10.1016/j.micinf.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 44. Miller CM, Zakrzewski AM, Ikin RJ, Boulter NR, Katrib M, Lees MP, Fuller SJ, Wiley JS, Smith NC. 2011. Dysregulation of the inflammatory response to the parasite, Toxoplasma gondii, in P2X7 receptor-deficient mice. Int. J. Parasitol. 41:301–308. 10.1016/j.ijpara.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 45. Wen H, Miao EA, Ting JP. 2013. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39:432–441. 10.1016/j.immuni.2013.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, Kimura T, Okamoto T, Okuyama M, Kayama H, Nagamune K, Takashima S, Matsuura Y, Soldati-Favre D, Takeda K. 2011. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J. Exp. Med. 208:1533–1546. 10.1084/jem.20101660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Niedelman W, Sprokholt JK, Clough B, Frickel EM, Saeij JP. 2013. Cell death of interferon-gamma stimulated human fibroblasts upon Toxoplasma gondii infection induces early parasite egress and limits parasite replication. Infect. Immun. 81:4341–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW, Macmicking JD, Sibley LD. 2013. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 9:e1003320. 10.1371/journal.ppat.1003320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fruth IA, Arrizabalaga G. 2007. Toxoplasma gondii: induction of egress by the potassium ionophore nigericin. Int. J. Parasitol. 37:1559–1567. 10.1016/j.ijpara.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. 2012. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8:e1002638. 10.1371/journal.ppat.1002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. 2010. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 6:e1000906. 10.1371/journal.ppat.1000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Binder EM, Kim K. 2004. Location, location, location: trafficking and function of secreted proteases of Toxoplasma and Plasmodium. Traffic 5:914–924. 10.1111/j.1600-0854.2004.00244.x [DOI] [PubMed] [Google Scholar]
- 53. Choi WY, Nam HW, Youn JH. 1989. Characterization of proteases of Toxoplasma gondii. Kisaengchunghak Chapchi 27:161–170 [DOI] [PubMed] [Google Scholar]
- 54. Dou Z, Carruthers VB. 2011. Cathepsin proteases in Toxoplasma gondii. Adv. Exp. Med. Biol. 712:49–61. 10.1007/978-1-4419-8414-2_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dou Z, Coppens I, Carruthers VB. 2013. Non-canonical maturation of two papain-family proteases in Toxoplasma gondii. J. Biol. Chem. 288:3523–3534. 10.1074/jbc.M112.443697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim K. 2004. Role of proteases in host cell invasion by Toxoplasma gondii and other Apicomplexa. Acta Trop. 91:69–81. 10.1016/j.actatropica.2003.11.016 [DOI] [PubMed] [Google Scholar]
- 57. Shea M, Jäkle U, Liu Q, Berry C, Joiner KA, Soldati-Favre D. 2007. A family of aspartic proteases and a novel, dynamic and cell-cycle-dependent protease localization in the secretory pathway of Toxoplasma gondii. Traffic 8:1018–1034. 10.1111/j.1600-0854.2007.00589.x [DOI] [PubMed] [Google Scholar]
- 58. Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477:592–595. 10.1038/nature10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600. 10.1038/nature10510 [DOI] [PubMed] [Google Scholar]
- 60. Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317–327. 10.1016/j.immuni.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 61. Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP, Koller BH. 2012. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J. Immunol. 189:2006–2016. 10.4049/jimmunol.1201065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, Cengia LH, Henley KJ, Collinge JE, Kastner DL, Feigenbaum L, Hilton DJ, Alexander WS, Kile BT, Croker BA. 2012. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37:1009–1023. 10.1016/j.immuni.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- 64. Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. 2005. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect. Immun. 73:695–702. 10.1128/IAI.73.2.695-702.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jensen KD, Wang Y, Wojno ED, Shastri AJ, Hu K, Cornel L, Boedec E, Ong YC, Chien YH, Hunter CA, Boothroyd JC, Saeij JP. 2011. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9:472–483. 10.1016/j.chom.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, Leppla SH, Grigg ME, Saeij JPJ, Moayeri M. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Type II parasites activate the inflammasome without inducing cell death. BMDMs were primed with 100 ng/ml LPS or left unstimulated for 2 h and subsequently infected with type II parasites (Pru; MOI, 0.4) for 24 h. (A) Quantification of IL-1β in supernatants was performed using ELISA. (B) Cell viability of infected cells in panel A was determined at different time points using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. Download
Type I and type IV parasites induce cleavage of pro-IL-1β. C57BL/6 BMDMs were primed with 100 ng/ml LPS for 2 h and infected with type I (RH) or type IV (MAS) parasites (MOI, 5) for 24 h. Western blot analysis was performed on concentrated supernatant (25-fold), probing for pro-IL-1β (37 kDa) and active IL-1β (17 kDa). Download
Type II parasites activate the Nlrp3 inflammasome. IL-1β secretion from BMDMs prepared from wild-type C57BL/6 mice (blue) or C57BL/6 mice lacking Nlrp1b (red), Nlrp3 (green), or Nlrp1b and Nlrp3 (orange) primed for 4 h and then infected with type II parasites (Pru; MOI, 0.8) for 24 h. The figure represents one experiment. Error bars, +standard deviations. Download