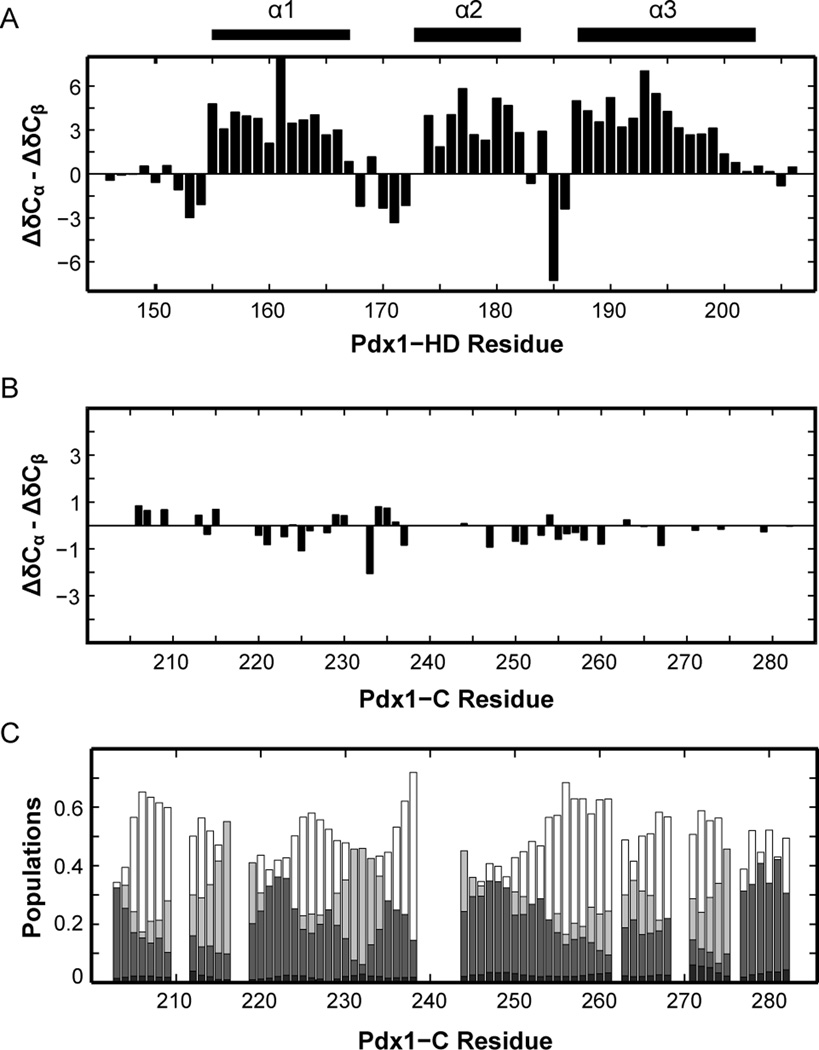
Figure 5.
Secondary chemical shifts of Pdx1-C indicate that this protein adopts an extended coil-like structure. (A) For comparison, the secondary structure of Pdx1-HD (BMRB accession number 19227) is shown as the difference between 13Cα and 13Cβ secondary chemical shifts, indicating the presence of an α-helix when long stretches of positive values are encountered. The secondary structure from the co-crystal of Pdx1 with a consensus DNA duplex is represented by bars above the figure for comparison. (B) Pdx1-C displays virtually no tendency towards secondary structure, based on the difference between 13Cα and 13Cβ secondary chemical shifts. (C) δ2D calculations for Pdx1-C (see Materials and Methods) provide additional insight into the nature of the extended structure found. For each residue, α-helix is shown in black, extended-β in dark grey, polyproline II in light grey, and coil structure as white (non-filled) bars.