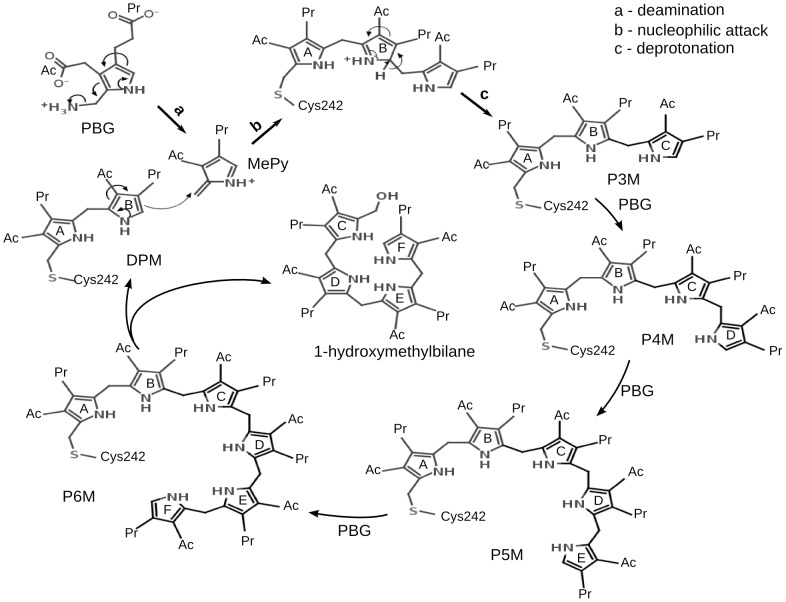
Figure 1. Mechanism of tetrapyrrole chain elongation catalyzed by PBGD.
The scheme shows the deamination of porphobilinogen (PBG) to methylene pyrrolenine (MePy), the nucleophilic attack by the B ring of dipyrromethane (DPM) on MePy forming an intermediate that undergoes deprotonation to form a tripyrrole moiety (P3M). Subsequent additions of PBG elongates the chain to form tetrapyrrole (P4M), pentapyrrole (P5M) and hexapyrrole (P6M) moieties. In the last step, the tetrapyrrole product, 1-hydroxymethylbilane (HMB) is hydrolyzed from DPM. The rings of the elongating pyrrole chain are labeled as A, B, C, D, E and F starting from the pyrrole ring covalently attached to C242. The acetate and propionate side-groups of the pyrroles are denoted by -Ac and -Pr, respectively.