FIGURE 5.
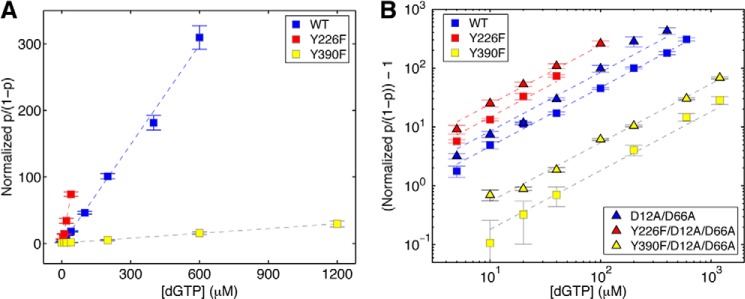
Complementary dNTP binding affinities of Φ29 DNAP mutants. The normalized p/(1 − p) (where p is the probability of post-translocation state occupancy, and the normalized p/(1 − p), defined as the value of p/(1 − p) in the presence of a given concentration of dNTP, divided by the value for p/(1 − p) for the same Φ29 DNAP-DNA complex at 0 μm dGTP (7)) is plotted (A) as a function of dGTP concentration for complexes formed between wild type, Y226F, and Y390F Φ29 DNAP. In B, (normalized p/(1 − p)) − 1 is plotted on a log scale as a function of dGTP concentration for complexes formed between the wild type, Y226F, Y390F, D12A/D66A, Y226F/D12A/D66A, or Y390F/D12A/D66A Φ29 DNAP and DNA1. Complexes were captured at 180 mV. Plot symbols for each of the enzymes are given in the legend to Fig. 4. Error bars, S.E. Each data point was determined from 15–30 ionic current time traces for individual captured complexes; each time trace had a duration of 5–10 s.
