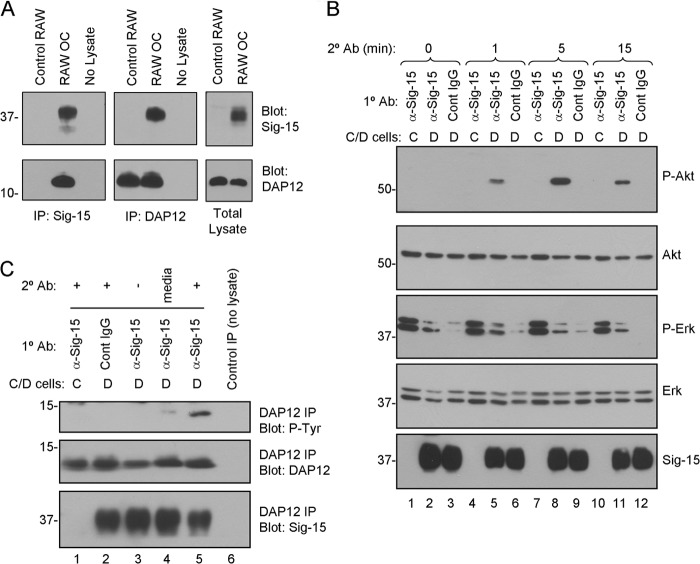
FIGURE 6.
Siglec-15 and DAP12 form a complex in osteoclasts, and Siglec-15 clustering induces DAP12 phosphorylation and Akt signaling. A, co-immunoprecipitation (Co-IP) of DAP12 and Siglec-15. Protein lysates were prepared from non-differentiated RAW264.7 cells (control RAW) or RAW264.7-derived osteoclasts. Siglec-15 (left panels) and DAP12 (middle panels) immunoprecipitates as well as total lysates (right panels) were analyzed by Western blotting with Siglec-15 and DAP12 antibodies. The no lysate IPs were performed using fresh lysis buffer. B, analysis of cell signaling induced by Siglec-15 clustering. Control (C) or differentiated (D) RAW264.7 cells were treated with primary antibody (anti-Siglec-15 or control human IgG) at 4 °C followed by a secondary cross-linking antibody for the indicated times at 37 °C. Total lysates were analyzed by Western blotting with the indicated antibodies. C, DAP12 phosphorylation following Siglec-15 cross-linking. RAW264.7 cells were treated as in B for 5 min with secondary antibody (lanes 1, 2, and 5). As additional controls, some cells were lysed immediately after primary antibody incubation (lane 3) and for others, the secondary antibody was omitted from the 37 °C incubation media (lane 4). Protein extracts were prepared, and DAP12 immunoprecipitates were analyzed by blotting with anti-phosphotyrosine, anti-DAP12, and anti-Siglec-15 antibodies.