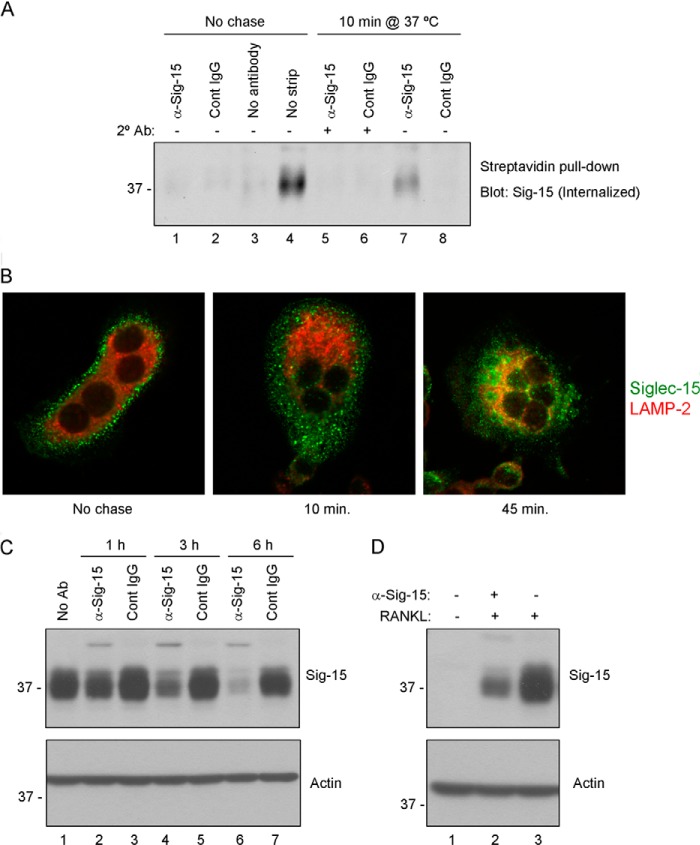
FIGURE 7.
Antibody-induced internalization and lysosomal degradation of Siglec-15 in osteoclasts. A, internalization of biotinylated Siglec-15. RAW264.7-derived osteoclast cell-surface proteins were labeled with a disulfide-linked biotinylation reagent. Cells were then treated with a combination of primary (anti-Siglec-15 B02 or control IgG) antibodies (at 4 °C) followed by secondary cross-linking antibodies (10 min at 37 °C, lanes 5 and 6), or with primary antibody alone (10 min at 37 °C, lanes 7 and 8). Control cells were incubated with B02, control IgG, or no antibody at 4 °C (lanes 1–3) without a 37 °C chase. After these antibody treatments, remaining cell-surface biotin was stripped with a reducing agent; for one sample, as an additional control (lane 4), this stripping step was omitted. Internalized, biotinylated proteins were collected from cell lysates by streptavidin immunoprecipitation, and Siglec-15 was detected by Western blotting. B, characterization of Siglec-15 endocytosis by confocal microscopy. RAW264.7-derived osteoclasts were cold-loaded with Siglec-15 antibody and either fixed immediately (No chase) or incubated in fresh, warm media for 10 or 45 min prior to fixation. Cells were then permeablized and stained with anti-LAMP2 and anti-human IgG (to detect internalized Siglec-15). C, Siglec-15 protein levels decrease rapidly following antibody treatment. Differentiated RAW264.7-derived osteoclasts were treated for the indicated times with anti-Siglec-15 or a control human IgG. Protein lysates were analyzed by Western blotting with the indicated antibodies. D, RAW264.7 differentiated in the presence of Siglec-15 antibodies show decreased Siglec-15 protein levels. Protein lysates of RAW264.7 cells, either non-differentiated (− RANKL) or differentiated (+ RANKL) in media with or without anti-Siglec-15 were analyzed by Western blotting with the indicated antibodies.