FIGURE 5.
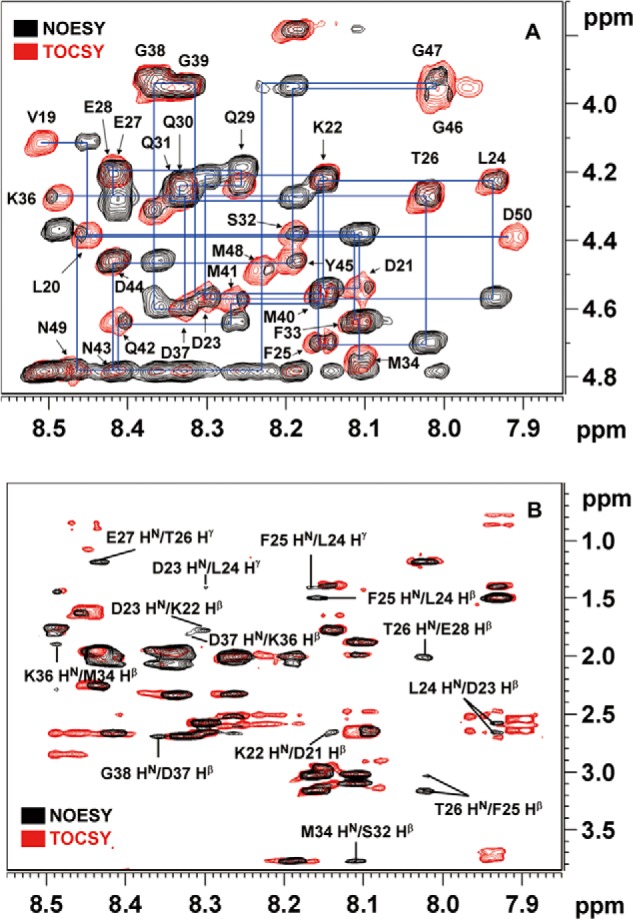
Chemical shift assignments and conformational analysis of the A17-N peptide by two-dimensional TOCSY and NOESY. A, sections of the 1HN-1Hα region of the NOESY and TOCSY spectra of the 33-mer A17-N peptide that binds to A27. The 1H chemical shift assignments were successfully determined using the inter- and intra-residual correlations in these spectra (supplemental Table S1). B, sections of 1HN-1Hβ and 1HN-1Hγ of the A17-N NOESY and TOCSY spectra. Consecutive inter-residual (i, i + 1) NOE signals were resolved as follows: between the 1Hβ of Asp21 and the 1HN of Lys22; between the 1Hβ of Lys22 and the 1HN of Asp23; between the 1HN of Asp23 and the 1Hγ of Leu24; between the 1Hβ of Asp23 and the 1HN of Leu24; between the 1Hβ of Leu24 and the 1HN of Phe25; between the 1Hγ of Leu24 and the 1HN of Phe25; between the 1Hβ of Phe25 and the 1HN of Thr26; between the 1Hγ of Thr26 and the 1HN of Glu27; between the 1Hβ of Gln30 and the 1HN of Gln31; and between the 1Hβ of Lys36 and the 1HN of Asp37. Additionally, (i, i + 2) NOE signals were found between the 1HN of Thr26 and the 1Hβ of Glu28, between the 1Hβ of Ser32 and the 1HN of Met34, and between the 1Hβ of Met34 and the 1HN of Lys36.
