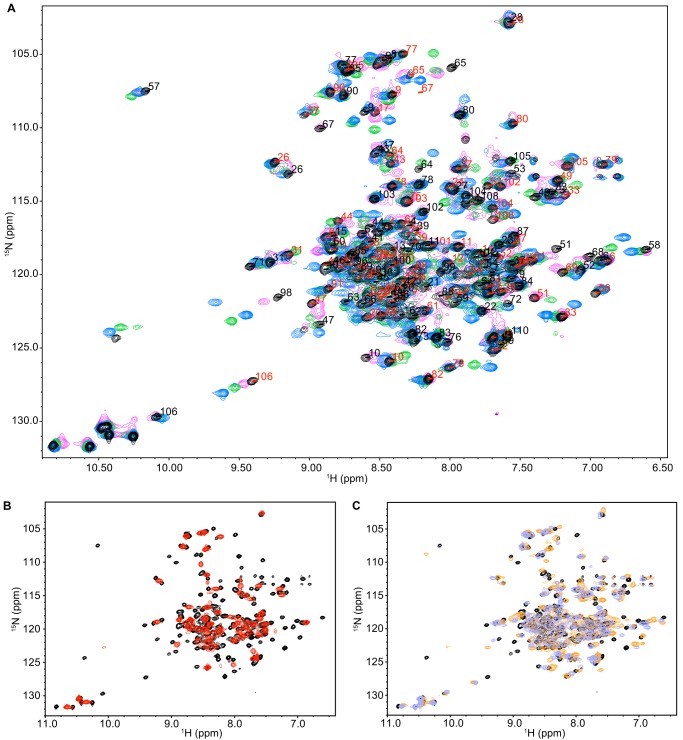
FIGURE 4.
NMR spectra of EmrE bound to the tetrahedral and planar substrates indicate the same overall protein structure but varying dynamics. A, 15N/1H TROSY HSQC spectra of EmrE bound to EtTPP+ (magenta), TPP+ (black), DPhTPP+ (blue), and MBTPP+ (green), are similar, with two peaks per residue corresponding to slow exchange of the asymmetric dimer. The assignments for TPP+-bound EmrE are displayed for states A (red) and B (black). B, 15N/1H TROSY HSQC spectrum of EmrE bound to MeTPP+ (red) has a single set of peaks at the average chemical shift, revealing faster conformational exchange of EmrE bound to this ligand than TPP+ (black). C, overlay of TPP+-bound EmrE (black) with EmrE bound to the two planar ligands, PP2+ (DMPC bicelles; orange) and DQ2+ (lavender). All spectra were collected identically at pH 7 and 45 °C, with 2-s recycle delay for all ligands except for MeTPP+ (6-s recycle delay).