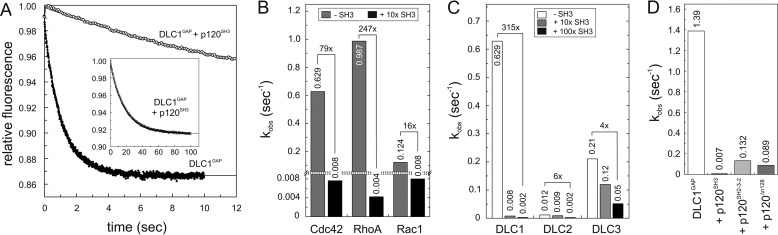
FIGURE 4.
p120SH3 as a potent inhibitor of the DLC GAP function. A, kinetics of the tamraGTP hydrolysis reaction of Cdc42 (0.2 μm) stimulated by DLC1GAP (5 μm) was reduced in the presence of a 10-fold excess of p120SH3 (50 μm). The complete reaction is shown in the inset. B, DLC1GAP activities toward Cdc42, RhoA, and Rac1, measured under the same conditions as in A, are strongly inhibited by p120SH3. For convenience, the kobs values are given above the bar charts. C, DLC3GAP (5 μm) was not inhibited by p120SH3 (50 and 500 μm) as efficiently as DLC1GAP and DLC2GAP (5 μm, respectively). D, p120SH2-3-2 and p120Δn128 (40 μm) inhibited the activity of DLCGAP (10 μm) but not as efficiently as p120SH3 (40 μm).