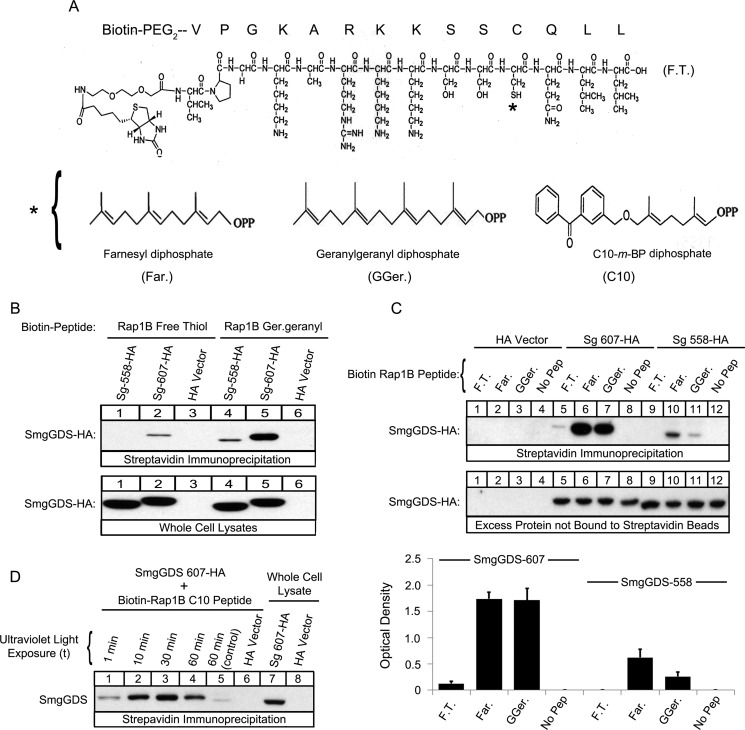
FIGURE 5.
SmgGDS-607 binds directly to both the prenylated and nonprenylated forms of a Rap1B C-terminal peptide, and SmgGDS-558 binds to the prenylated form of the peptide. A, the structure of the free thiol Rap1B peptide is shown, where the asterisk indicates the farnesyl (left panel), geranylgeranyl (middle panel), or C10-m-BP (right panel) group. The C10 group contains the photoactive benzophenone-containing residue, which allows for photo-cross-linking to a substrate. B and C, the indicated Biotin-PEG-Rap1B peptides in the free thiol (F.T.), farnesylated (Far.), or geranylgeranylated (GGer.) forms were prebound to streptavidin beads and allowed to interact with lysates of HEK-293T cells expressing either SmgGDS-607-HA, SmgGDS-558-HA, or HA vector. The bound complexes, whole cell lysates (B), and excess protein not bound to the streptavidin beads (C) were subjected to ECL-Western blotting using HA antibody. The results are representative of three independent experiments. D, Biotin-PEG-Rap1B C10-m-BP peptides were allowed to interact with HEK-293T cell lysates containing either SmgGDS-607-HA or HA vector and exposed to increasing times of UV light (1, 10, 30, and 60 min). The control sample was wrapped in tin foil and exposed to UV light for 60 min. Streptavidin beads were used to pull down the complexes of SmgGDS-607-HA cross-linked to the biotinylated Rap1B peptide, and then the complexes and whole cell lysates were subjected to ECL-Western blotting using HA antibody. The results are representative of three independent experiments.