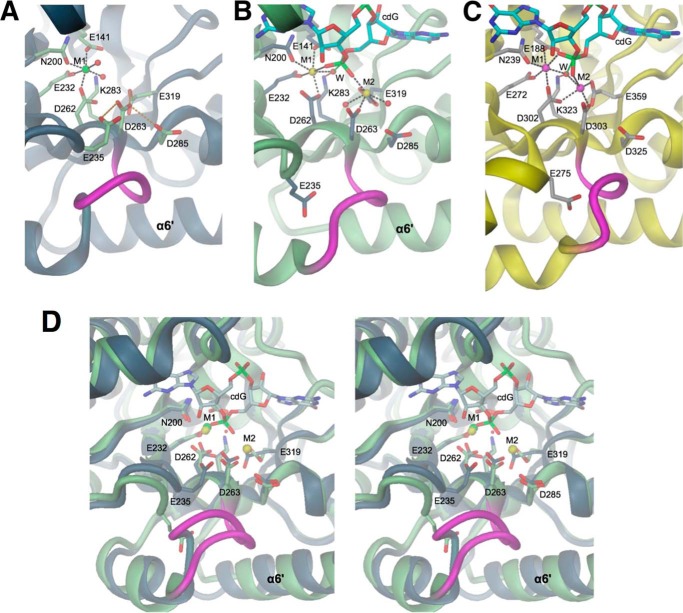
FIGURE 4.
EAL active site structures in the absence and presence of cdG substrate. Divalent cations are colored in green (Mg2+), yellow (Ca2+), or magenta (Mn2+). A, YahA-EAL·Mg2+, B, YahA-EAL·cdG·Ca2+, and C, BlrP1·cdG/Mn2+ (PBD code 3GG0) (12). Water molecules are represented as red spheres, cation coordination bonds by black broken lines, and loop β5-α5 is highlighted in magenta. In A, Asp-263 is H-bonded (orange broken lines) to surrounding carboxylic side chains. In B and C, the hydrolytic water (W) is in-line with the scissile O3′-P bond of the cdG substrate (shown in full with cyan carbons). Note that the location of the “anchoring” glutamate (Glu-235 in YahA, Glu-275 in BlrP1) is distinct in the binary and ternary complexes. D, stereoview of the superposition of YahA-EAL in complex with Mg2+ (ribbon and carbon atoms in gray) and cdG·Ca2+ (ribbon and carbons atom in light green).