FIGURE 8.
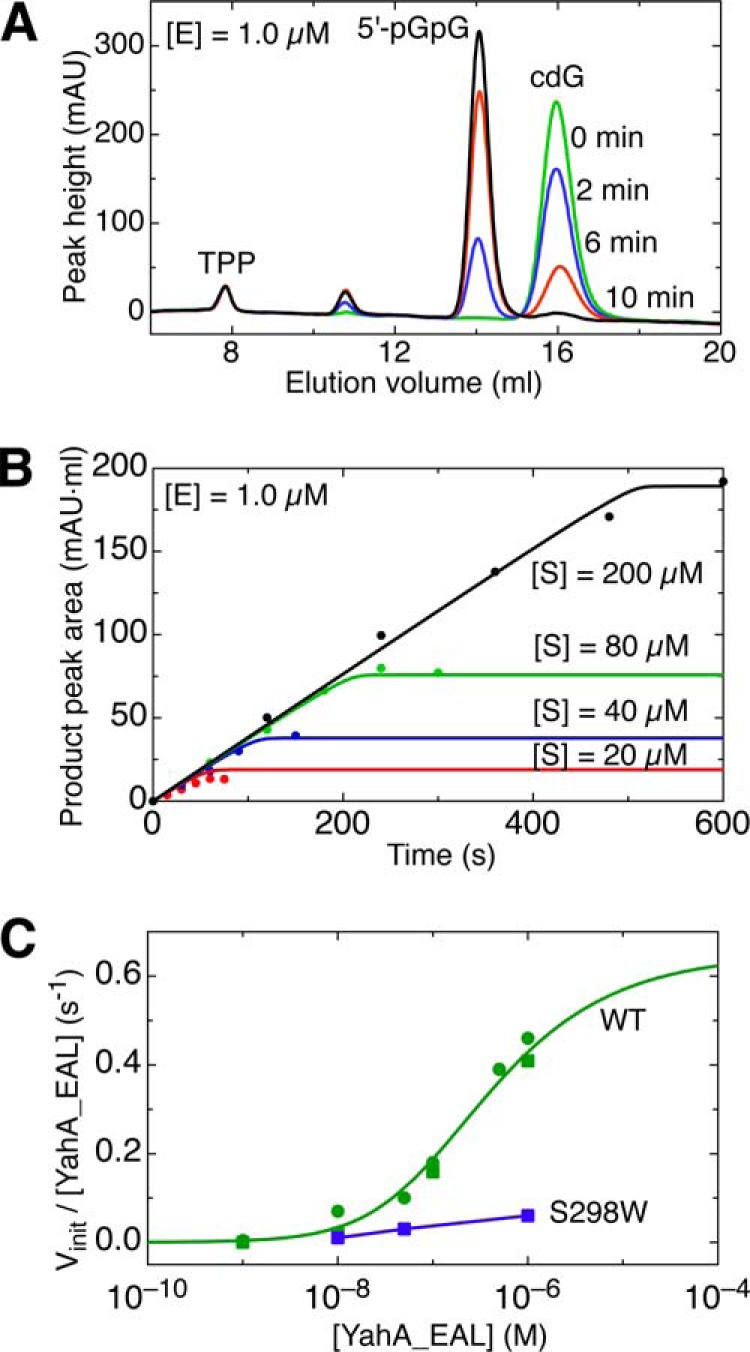
Catalytic activity of the YahA-EAL domain. A, FPLC chromatograms showing time-dependent conversion of cdG to pGpG. CdG (200 μm) was incubated for the indicated time spans with 1 μm YahA-EAL. B, progress curves of pGpG production with the initial cdG substrate concentrations indicated. Data were fitted to a simple Michaelis-Menten kinetics model. C, specific activity vinit/[YahA-EAL] as a function of [YahA-EAL] concentration, acquired at saturating substrate concentration. For the wild-type protein (green), data points are shown from two separate experiments. The data were fitted (continuous line) to a simple monomer-dimer equilibrium model and indicate that the enzyme is inactive as monomer. The dimer interface mutant S298W (blue) is virtually inactive.
