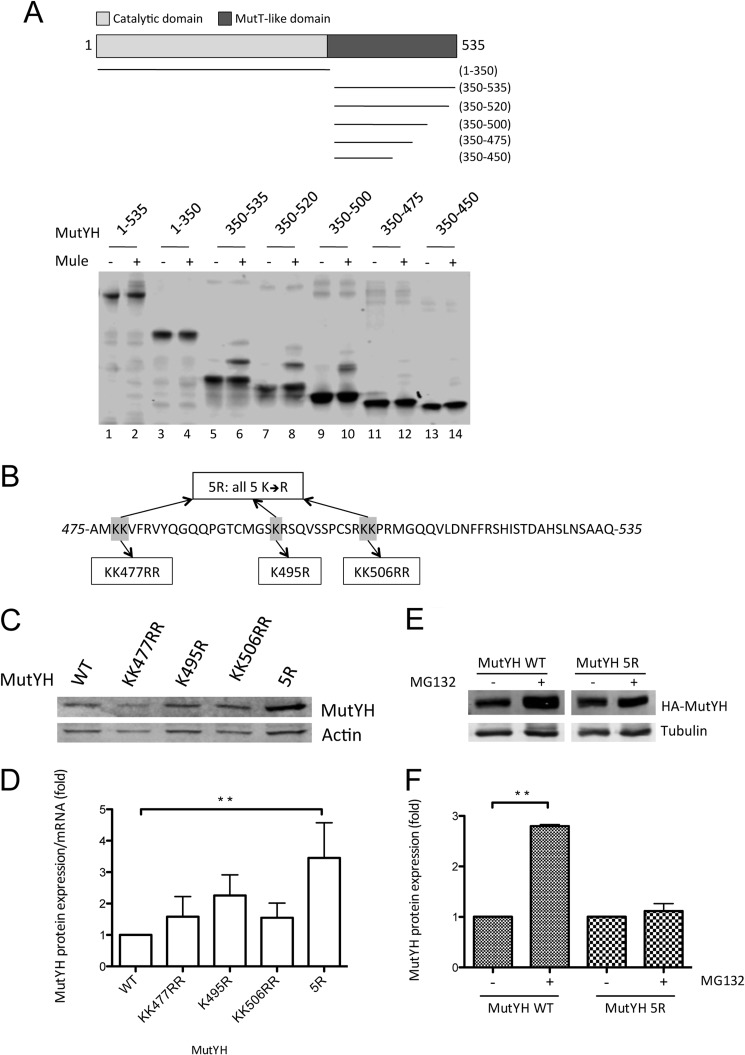
FIGURE 4.
MutYH is ubiquitinated between amino acids 475 and 535, and mutation of the five lysine residues located in this part stabilizes MutYH on the protein level. A, in vitro ubiquitination of different recombinant and purified MutYH deletion constructs, as depicted in the upper part. B, amino acid sequence of MutYH between positions 475 and 535. Highlighted are the lysine residues that were mutated into arginine. C, transfection of the MutYH point mutant constructs (as indicated) into HEK293T cells, analyzed by Western blotting. D, normalization of the mutant MutYH protein level as shown in C to the mRNA level of the respective expression construct (p < 0.004). The protein levels were normalized to actin and the mRNA levels to L28 mRNA. E, transfection of the MutYH WT and 5R construct into HEK293T cells. The cells were treated with 10 mm MG132 as indicated, 16 h prior to harvest. The samples were analyzed by Western blotting. F, quantification of the protein levels shown in E (p < 0.01). The MutYH levels were normalized to tubulin. Presented are the results of two independent experiments.