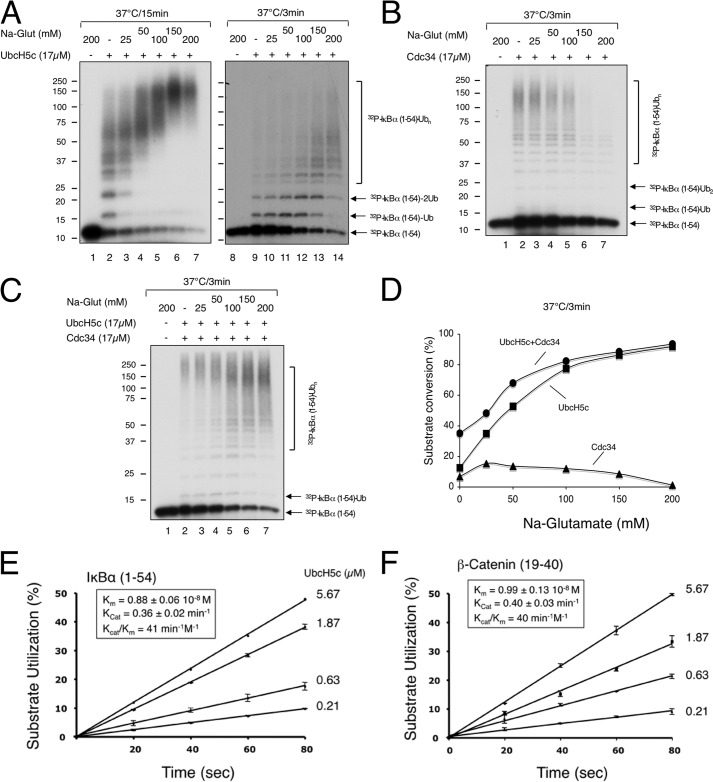
FIGURE 1.
Characterization of polyubiquitination of IκBα-(1–54) and β-catenin by SCFβTrCP2, UbcH5c, and Cdc34. A–D, sodium glutamate activates UbcH5c but inhibits Cdc34 activity. The effects of increasing concentrations of sodium glutamate (as indicated) on polyubiquitination of IκBα-(1–54) by SCFβTrCP2 in the presence of UbcH5c (panel A), Cdc34 (panel B), or both UbcH5c and Cdc34 (panel C). Note that sodium glutamate (Na-Glut), at 150 and 200 mm, affects gel migration such that the input substrate bands appear wider (panel B, lanes 1, 6, and 7). Phosphorimaging revealed no substrate utilization under these conditions. The detailed procedure is described under “Experimental Procedures” with incubation times as indicated. The reactions were quantified and shown graphically (panel D). E and F, kinetic parameters of polyubiquitination of IκBα-(1–54) and β-catenin by SCFβTrCP2 and UbcH5c. 32P-Labeled IκBα-(1–54) (2pmol) or β-catenin (2pmol) were incubated with SCFβTrCP2 (∼2pmol) for 10 min. Separate mixtures of UbcH5c∼Ub at differing concentrations (5.67, 1.87, 0.63, and 0.21 μm) of UbcH5c were incubated at 37 °C for 5 min. The two mixtures were combined, and reactions were stopped by the addition of SDS-loading buffer after 20, 40, 60, or 80 s. Substrate utilization of three independent data sets was calculated and shown graphically for the reaction of IκBα-(1–54) (panel E) or β-catenin (panel F). Km and Kcat were estimated by fitting to Michaelis-Menten kinetics and shown in the inset box.