FIGURE 2.
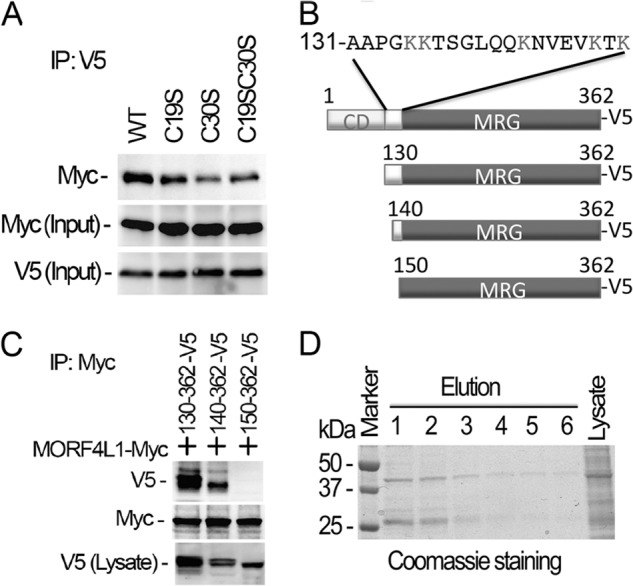
Deletion of the NH2 terminus of MORF4L1 ablates its homodimerization. A, MORF4L1 cysteine residue mutants tagged with V5 were coexpressed with Myc-tagged, wild-type MORF4L1 in the cells. The cell lysates were used for immunoprecipitation (IP) studies as indicated. Input was 10% of the cell lysate used in the studies. B, schematic presentation of the deletion mutations. C, NH2-terminal deletion mutants were expressed in MLE cells. The cell lysates were subjected to V5 immunoprecipitation, and the immunoprecipitates were analyzed by immunoblotting as indicated. NH2-terminal deletional mutant expression in the cells was checked by V5 immunoblotting. D, purified NH2-terminal truncated MORF4L1 forms a dimer efficiently. NH2-terminal truncated MORF4L1 was expressed and purified. Elution products were separated by electrophoresis, and the proteins were visualized by Coomassie Blue stain. These data are representative of three separate experiments.
