FIGURE 5.
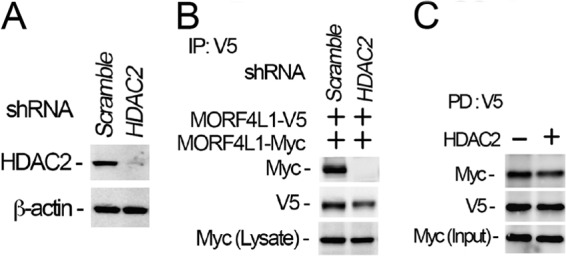
HDAC2 is necessary for MORF4L1 self-assembly. A, HDAC2 was depleted in cells by introducing shRNA retroviral constructs for 48 h. Cell lysates were analyzed with HDAC2 immunoblotting. A scrambled retroviral plasmid was used as a shRNA specificity control. β-actin was used as a loading control. B, HDAC2 was first knocked down by shRNA for 24 h in the cells, and then V5- or Myc-tagged MORF4L1 were expressed for another 24 h. Cell lysates were analyzed by V5 immunoprecipitation (IP), followed by immunoblotting. C, V5- and Myc-tagged MORF4L1 and HDAC2 were synthesized in vitro and the MORF4L1 recombinant proteins were mixed with V5 antibody-conjugated agarose beads in the presence or absence of HDAC2 overnight. The washed beads were analyzed by immunoblotting as indicated. Input was 10% of the recombinant protein used in the pulldown (PD) assays. These data are representative of three separate experiments.
