FIGURE 7.
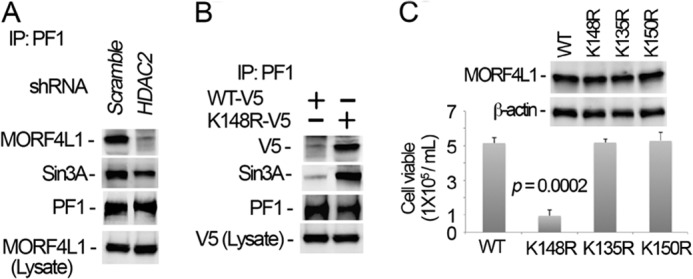
Dimerization augments MORF4L1 complex formation and cellular viability. A, HDAC2 was depleted in cells by introducing shRNA retroviral constructs for 48 h. Cell lysates were immunoprecipitated (IP) with PF1 antibody, and the precipitates were analyzed by immunoblotting. A scrambled retroviral plasmid was used as a shRNA specificity control. B, wild-type and K148R mutant MORF4L1 plasmids were coexpressed in cells for 48 h. Cell lysates were used for PF1 immunoprecipitation, followed by immunoblot analysis. Wild-type and K148R mutant MORF4L1 plasmids in the cells were validated by V5 immunoblotting. C, wild-type and K135R, K148R, and K150R mutant MORF4L1 plasmids were expressed in the cells for 48 h. Viable cells were counted in each transfected cell line. The expression levels of the proteins were analyzed by immunoblotting. The data are representative of three separate experiments.
