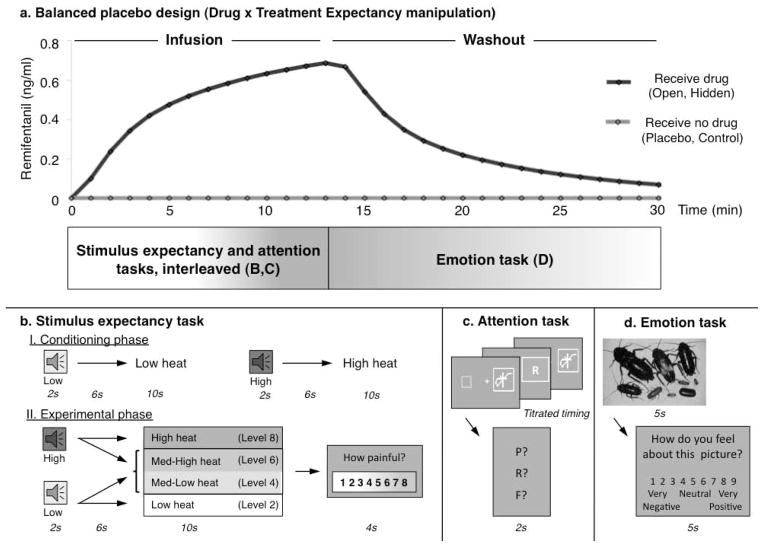
Figure 1. Experimental Design.
a) We used a balanced placebo design, wherein participants received remifentanil intravenously during two runs (Red; Open and Hidden administration) and received no remifentanil during two runs (Blue: Placebo and Control conditions). The stimulus expectancy task and the attention task were presented during drug infusion, while the emotion task was performed during the washout period. We estimated performance as a function of predicted drug concentration over time. The figure illustrates predicted drug concentration for one participant. b) Prior to the main experiment, participants went through a conditioning phase designed to manipulate stimulus expectancies, in which two auditory cues were followed by low or high intensity stimulation. During the main experiment, each cue was equally likely to be followed by its predicted level (level 2 or level 8 on an 8-point scale) or two levels of heat calibrated to elicit ratings of medium pain (level 4 or level6). Participants provided pain reports on each trial using a continuous visual analogue scale. c) Following each trial of the stimulus expectancy task (i.e. after participants provided pain ratings), participants performed three attention task trials. A target letter was presented in the midst of a chain of rapidly changing symbols, with durations determined through a titration phase. Following stimulus presentation, participants reported the letter they had seen on that trial using a forced-choice response. d) The emotion task was presented during the washout period. Participants saw negative, neutral, and positive images from the International Affective Pictures Set (Lang et al, 1998) and rated how negative or positive they felt in response to the image, using a 9-point scale.