Abstract
Aims/hypothesis
Insulin-sensitive tissues (muscle, liver) of individuals with obesity and type 2 diabetes mellitus are in a state of low-grade inflammation, characterised by increased Toll-like receptor (TLR) expression and TLR-driven signalling. However, the cause of this mild inflammatory state is unclear. We tested the hypothesis that a prolonged mild increase in plasma NEFA will increase TLR expression and TLR-driven signalling (nuclear factor κB [NFκB] and mitogen-activated kinase [MAPK]) and impair insulin action in muscle of lean healthy individuals.
Methods
Twelve lean, normal-glucose-tolerant participants were randomised to receive a 48 h infusion (30 ml/h) of saline or Intralipid followed by a euglycaemic–hyperinsulinaemic clamp. Vastus lateralis muscle biopsies were performed before and during the clamp.
Results
Lipid infusion impaired insulin-stimulated IRS-1 tyrosine phosphorylation and reduced peripheral insulin sensitivity (p < 0.01). The elevation in circulating NEFA increased expression of TLR3, TLR4 and TLR5, and several MAPK (MAPK8, MAP4K4, MAP2K3) and inhibitor of κB kinase-NFκB (CHUK [IKKA], c-REL [REL] and p65 [RELA, NFKB3,p65]) signalling genes (p < 0.05). The lipid infusion also increased extracellular signal-regulated kinase (ERK) phosphorylation (p < 0.05) and tended to reduce the content of nuclear factor of light polypeptide gene enhancer in B cells inhibitor α (p = 0.09). The muscle content of most diacyglycerol, ceramide and acylcarnitine species was unaffected. In summary, insulin resistance induced by prolonged low-dose lipid infusion occurs together with increased TLR-driven inflammatory signalling and impaired insulin-stimulated IRS-1 tyrosine phosphorylation.
Conclusions/interpretation
A sustained, mild elevation in plasma NEFA is sufficient to increase TLR expression and TLR-driven signalling (NFκB and MAPK) in lean individuals. The activation of this pathway by NEFA may be involved in the pathogenesis of insulin resistance in humans.
Keywords: Acylcarnitine, Ceramide, Diacylglycerol, Inflammation, Insulin resistance
Introduction
Over the past three decades a large body of evidence has implicated NEFA in the pathogenesis of skeletal muscle insulin resistance. For example, most individuals with obesity and type 2 diabetes have elevated plasma NEFA levels, which correlate with the severity of insulin resistance [1]. In insulin-resistant individuals, pharmacological reduction of plasma NEFA concentration with anti-lipolytic drugs improves insulin sensitivity [2]. Accordingly, acute experimental elevation in plasma NEFA levels (by systemic lipid infusion) rapidly induces skeletal muscle insulin resistance in lean healthy individuals [3, 4].
Different theories have been proposed to explain how excess NEFAs cause insulin resistance in muscle. Studies in cultured cells and animals have shown that toxic metabolites of NEFAs and triacylglycerols, such as ceramides and diacylglycerols (DAGs), interfere with the insulin signalling pathway (i.e. lipotoxicity). An alternative theory proposes that NEFAs impair glucose metabolism by stimulating cell-surface receptors of the Toll-like receptor (TLR) family (TLR2 and TLR4) [5, 6]. Common to both models (lipotoxicity and TLRs) is the stimulation of downstream pro-inflammatory signalling pathways, including the inhibitor of kappa B (IκB) kinase (IKK)-nuclear factor κB (NFκB) and the mitogen-activated kinase (MAPK) axes. The activation of IKK and the MAPKs (extracellular signal-regulated kinase [ERK], c-Jun N-terminal kinase [JNK] and p38) impairs insulin signal transduction [7]. These inflammatory pathways also increase the transcription of genes whose products (i.e. TNF-α and IL-6) are implicated in insulin resistance [7]. Notably, there is some evidence suggesting that TLR4 mediates NEFA-induced ceramide biosynthesis, raising the possibility that both proposed mechanisms of insulin resistance (intracellular lipids and TLR-mediated) are inextricably linked [8].
Inflammatory mediators in muscle, such as TLR4 [9], ERK [10], p38 [11], JNK [12] and NFκB [9], are increased in obesity and type 2 diabetes. However it is unclear whether the upregulation of these inflammatory mediators is a direct result of elevated plasma NEFA levels in humans in vivo. In addition, some studies in lean healthy humans have shown that the insulin resistance caused by acute lipid infusion occurs in association with increased muscle content of ceramides and DAGs [4, 13, 14], whereas other studies have not found changes in muscle lipid content [15–17]. Similarly, some [18–20] but not all [13, 15] studies have demonstrated impaired proximal insulin signalling following lipid infusion in humans. The discrepant findings are likely a result of the large variation in the lipid infusion protocols, most of them short (≤ 6 h) and some involving high lipid infusion rates and/or co-administration of heparin [3], which creates supraphysiological plasma NEFA levels by activating endothelial lipoprotein lipase. The aim of the present study was to test the hypothesis that a prolonged (48 h), mild increase in plasma NEFA will induce an inflammatory response in muscle of lean healthy individuals. We focused our analysis on TLR expression and TLR-driven pathways (NFκB and MAPK), considering the substantial body of evidence generated in recent years about their potential role in the pathogenesis of insulin resistance [8, 9, 21–23]. In addition, we tested whether the low-dose lipid infusion and its potential effect on inflammatory pathways led to impairments in the phosphorylation of insulin signalling intermediaries.
Methods
Participants
Twelve healthy, lean (BMI < 26 kg/m2), non-smoking, normal-glucose-tolerant, community-dwelling individuals (five men/seven women), without a family history of diabetes mellitus (first-degree relatives), were studied. Participants ranged from 18 to 60 years of age. Each participant underwent a physical examination, screening laboratory tests and an OGTT to document normal glucose tolerance status. Before enrolment, all participants maintained a stable body weight for more than 3 months (< 2% change). Participants were sedentary (performed < 30 min of exercise per week) and did not take medications known to affect glucose metabolism. Female participants were pre-menopausal and were studied during the follicular phase. This study was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio. All participants provided their informed written consent.
OGTT
After an overnight fast, participants ingested a solution containing 75 g glucose. Plasma glucose and insulin concentrations were measured at baseline and at 2 h. The HOMA of insulin resistance (HOMA-IR) was calculated from the fasting plasma glucose and insulin concentrations as described [24].
Saline/lipid infusion
Within 30 days of the OGTT, participants were admitted to our Diabetes Research Unit located in the South Texas Veterans Health Care System. They were randomised to receive a 48 h infusion through an antecubital vein of either normal saline or Intralipid 20% (Baxter Healthcare, Deerfield, IL, USA) at a rate of 30 ml/h. During this time, participants ingested a diet, consisting of 55% carbohydrate, 25% fat and 20% protein, providing an equivalent energy intake to that of their habitual diet. Participants consumed identical meals during each hospital admission. Complete food intake was confirmed by a research nurse after each meal. No exercise beyond habitual walking was permitted.
Muscle biopsy and insulin clamp
On the third day of admission, after an overnight fast, participants underwent a vastus lateralis muscle biopsy [25]. The tissue was rapidly frozen in liquid nitrogen and stored at −80° C. A 180 min euglycaemic–hyperinsulinaemic (80 mU m−2 min−1) clamp study was started 30 min after the biopsy. A second vastus lateralis muscle biopsy was obtained ~160 min after the start of the euglycaemic–hyperinsulinaemic clamp. The saline/lipid infusion was administered throughout the clamp, for a total of 48 h. Insulin-stimulated glucose metabolism (M value) was determined as the mean glucose infusion rate during the last 30 min of the clamp [26]. Participants who first received saline returned 4–8 weeks later to undergo the same procedures, with the exception that during the second hospitalisation they received the lipid infusion, and vice versa.
Plasma chemistry
Plasma insulin was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA, USA), glucose by the glucose oxidase method on an Analox analyser (Lunenburg, MA, USA) and HbA1c using a DCA2000 analyser (Bayer, Tarrytown, NY, USA). Plasma NEFA and triacylglycerol concentrations were determined using enzymatic assays (Wako, Nuess, Germany). Plasma IL-6 and TNF-α were measured using Multiplex immunobead assay technology (Millipore, Billerica, MA, USA) on a MAGPIX, xPONENT4.2 instrument (Luminex, Austin, TX, USA). Plasma fetuin-A was measured by quantikine human fetuin-A ELISA (R&D Systems, Minneapolis, MN, USA).
Quantitative RT-PCR
Analysis of the samples was performed using an RT Profiler PCR Array for Human TLR Signaling (SABiosciences, Frederick, MD, USA) on an ABI Prism 7900HT sequence detector (Applied Biosystems, Foster City, CA, USA). The threshold cycle (Ct) was calculated for each gene using Sequence Detection software, version 2.4 (Applied Biosystems). Ct data were uploaded into the data analysis template on the manufacturer’s website (www.sabiosciences.com/pcr/ arrayanalysis.php, accessed 8 August 2012). Gene expression was normalised to a panel of five housekeeping genes to determine the fold change in gene expression between saline and lipid infusion samples by the 2−ΔΔCt method.
Immunoblotting
Muscle was homogenised in lysis buffer (20 mmol/l Tris, pH 7.5, 5 mmol/l EDTA, 10 mmol/l Na3PO4, 100 mmol/l NaF, 2 mmol/l Na3VO4, 1% Nonidet P-40, 10 µmol/l leupeptin, 3 mmol/l benzamidine, 10 µg/ml aprotinin, 1 mmol/l phenylmethylsulfonyl fluoride). Proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated overnight with primary antibodies of interest. Antibodies to phospho-JNK (Thr183/Tyr185), JNK, ERK, phospho-p38 (Thr180/Tyr182), p38, IκBα, COX2, phospho-Akt (Ser473), Akt, phospho-GSK-3α/β (Ser21/9), GSK-3α, GSK-3α and phospho-AS160 (Ser588) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies to TLR4 (M-80), TLR2 (H-175) and NFκB p65 (H-286) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to IRS-1 and AS160 were obtained from Millipore and phospho-ERK (Thr185/tyr187) from Invitrogen (Carlsbad, CA, USA). Phospho-IRS-1 (Tyr612) was obtained from Sigma-Aldrich (St Louis, MO, USA). Coomassie staining (crude membrane extracts) verified equal protein loading across the gel lanes. Detection of primary antibodies was performed using an appropriate peroxidase-conjugated IgG, and protein signals were visualised using enhanced chemiluminescence reagents (GE Healthcare, Waukesha, WI, USA) by exposure to autoradiographic film. Quantification of immunoblots was performed using ImageQuant software (Molecular Dynamics, Fairfield, CT).
DAG and ceramide content
Concentrations of total DAG and ceramide in muscle were measured by thin-layer chromatography as previously described [27].
DAG and ceramide species content
The muscle content of ceramide and DAG species was measured in the Lipidomics Core of the University of South Carolina by high-performance liquid chromatography/mass spectrometry (LC-MS/MS), as previously described [28]
Acylcarnitine content
Muscle acylcarnitine content was measured by Lipomics Services, Metabolon (West Sacramento, CA, USA). Deuterium-labelled internal standards were added to muscle and the mixture was solubilised in methanol, followed by a crash extraction. The extracted mixture was injected onto an Atlantis HILIC Column connected to a Waters Xevo triple quadrupole mass spectrometer (Waters, Milford, MA, USA). The analytes were ionised via positive electrospray and the mass spectrometer was operated in the tandem mass spectrometry mode (ESI-MS/MS). The absolute concentration of acylcarnitine was determined by comparing the peak with that of the relevant internal standard.
Statistical analysis
All data are represented as the mean ± SE. The effect of the lipid infusion was evaluated using a paired t test or one-way ANOVA with Tukey–Kramer multiple comparisons test (GraphPad Software, San Diego, CA, USA).
Results
Characteristics of participants
Table 1 shows the baseline and clinical characteristics of the participants in this study. The participants were middle-aged, lean and had normal glucose tolerance.
Table 1.
Clinical and laboratory characteristics of participants
| Characteristic | Value |
|---|---|
| Sex (n, male/female) | 5/7 |
| Age (years) | 40 ± 3 |
| BMI (kg/m2) | 23.5 ±0.7 |
| Fasting plasma glucose (mmol/l) | 5.3 ± 0.1 |
| 2 h plasma glucose (mmol/l) | 5.6 ± 0.3 |
| Fasting plasma insulin (pmol/l) | 27.1 ± 4.9 |
| Fasting triacylglycerols (mmol/l) | 3.3 ± 0.5 |
| HbA1c (mmol/mol) | 35.7 ± 0.9 |
| HbA1c (%) | 5.4 ± 0.1 |
Data are means ± SE, n = 12
Effect of lipid infusion on plasma NEFA and insulin sensitivity
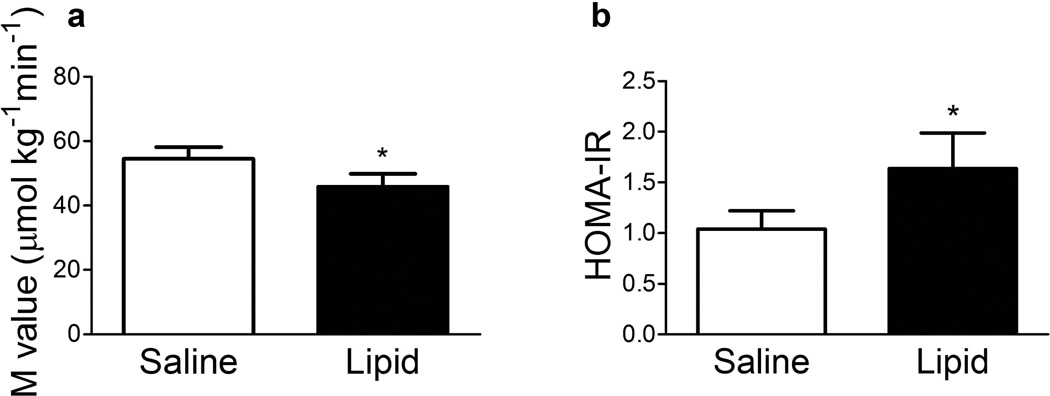
The lipid infusion increased plasma NEFA concentration from 355 ± 33 µmol/l (baseline) to 433 ± 39 µmol/l (24 h; p = 0.05) and 484 ± 24 µmol/l (48 h; p < 0.01) whereas saline did not significantly change plasma NEFA (baseline, 374 ± 26 µmol/l; 24 h, 354 ± 57 µmol/l; 48 h, 420 ± 25 µmol/l; p = NS). Administration of the lipid infusion resulted in a 17% reduction in peripheral insulin sensitivity (M) (Fig. 1a, p < 0.05). There was no difference between saline and lipid treatments in the mean plasma insulin concentration during the last 30 min of the clamp (150–180 min) (826 ± 49 vs 819 ± 63 pmol/l, respectively). Lipid infusion caused a 1.6-fold increase in the HOMA-IR index (Fig. 1b, p < 0.05), indicating decreased hepatic insulin sensitivity.
Fig. 1.
Effect of lipid infusion on insulin-stimulated glucose metabolism (M) (a) and HOMA-IR (b). Results are mean ± SE (n = 12). *p < 0.05 vs saline infusion
Effect of lipid infusion on inflammation-related genes
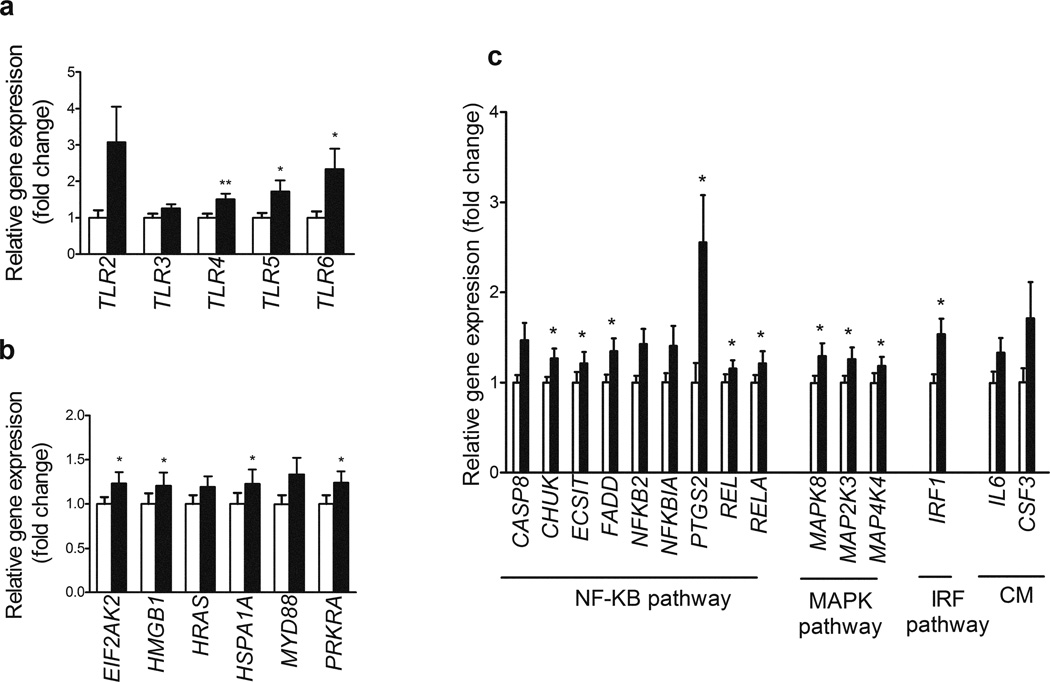
We used the TLR pathway PCR Array to test the hypothesis that lipid infusion would increase inflammatory signalling in muscle. All genes measured are shown in electronic supplementary material (ESM) Table 1. We found that 18 out of the 84 genes (21%) represented on the PCR array were significantly upregulated with lipid infusion when compared with saline (Fig. 2, p < 0.05). No genes were found to be significantly downregulated with lipid infusion. Of the 12 characterised TLRs, increased expression of three genes was observed following lipid infusion: TLR4 (50%), TLR5 (61%) and TLR6 (twofold). There was also a trend for expression of TLR2 (2.5-fold, p = 0.07) and TLR3 (30%, p = 0.06) to be increased following lipid infusion (Fig. 2a). Of the 26 TLR adaptors, interacting proteins and effectors represented on the PCR array, lipid infusion significantly increased the expression of four genes: EIF2AK2 (21%), HMGB1 (21%), HSPA1A (22%) and PRKRA (24%) (Fig. 2b). There was also a trend for HRAS (19%, p = 0.06) and MYD88 (28%, p = 0.08) expression to be increased following lipid infusion. Forty-six of the genes measured are known to be targets downstream of TLR signalling. Six genes of the NFκB signalling pathway were significantly upregulated following lipid infusion: CHUK (26%), ECSIT (24%), FADD (34%), PTGS2 (COX2) (2.3-fold), REL (16%) and RELA (20%) (Fig. 2c). There was also a trend for increased CASP8 (41%, p = 0.07), NFKB2 (37%, p = 0.06) and NFKBIA (34%, p = 0.08) expression following lipid infusion. The expression of three MAPK pathway-focused genes was increased following lipid infusion: MAPK8 (27%) and MAP2K3 (24%) and MAPK4K4 (39%) (Fig. 2c). One gene of the interferon regulatory factor (IRF) pathway (IRF1) was increased (53%) following lipid infusion (Fig. 2c). There was also a trend for two genes common to cytokine-mediated signalling pathways to be increased by lipid: IL6 (33%, p = 0.07 and CSF3 (54%, p = 0.07) (Fig. 2c)
Fig. 2.
Gene expression of TLRs (a), TLR adaptors, interactors and effectors (b) and genes involved in the NFκB, MAPK, IRF and cytokine-mediated signalling pathways (c) following saline (white bars) and lipid infusion (black bars). Only differentially expressed genes are shown. All genes measured are shown in ESM Table 1. Results are means ± SE (n = 10), fold change relative to saline infusion. *p < 0.05 and **p < 0.01 vs saline infusion
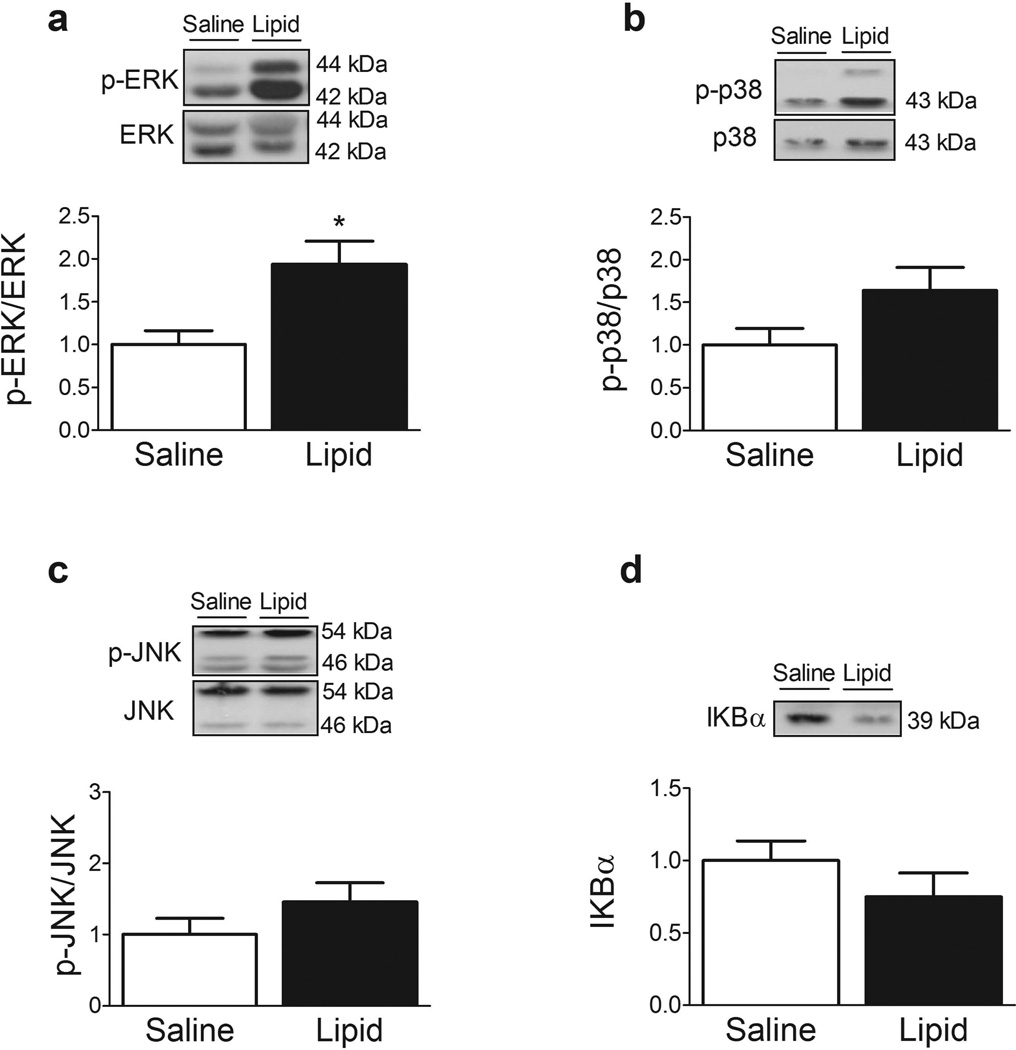
Effect of lipid infusion on inflammatory (MAPK and NFκB-IκBα) pathways
Compared with saline infusion, lipid significantly increased ERK phosphorylation by ~twofold (Fig. 3a, p < 0.05). In line with this finding, there was a trend for increased p38 (Fig. 3b, p = 0.08) and JNK (Fig. 3c, p = 0.13) phosphorylation following lipid infusion. We also evaluated the effect of the lipid infusion on the NFκB pathway. Phosphorylation by IKKβ targets IκBα for proteasomal degradation, which liberates NFκB for translocation into the nucleus where it promotes the expression of numerous genes. We identified a trend for reduced IκBα content in muscle following lipid infusion (Fig. 3d, p = 0.09), suggestive of increased flux through IKKβ-NFκB.
Fig. 3.
Effect of lipid infusion on phospho-ERK (a), phospho-p38 (b), phospho-JNK (c) and IκBα (d). Data are expressed as the ratio of phosphorylated to total protein, where appropriate, in arbitrary units as fold change compared with saline infusion. Representative immunoblots are shown. Results are means ± SE (n = 12). *p < 0.05 vs saline infusion
Effect of lipid infusion on ceramides, DAGs and acylcarnitines
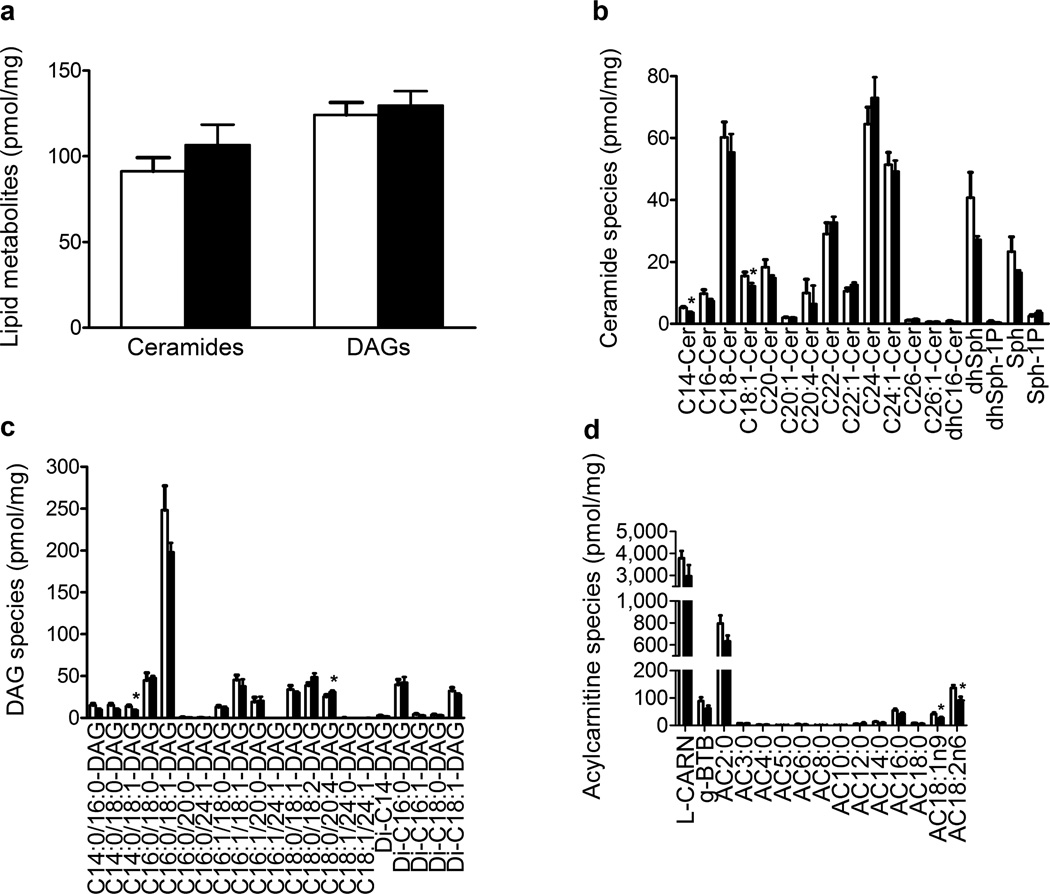
The ceramide content was higher in the muscle of 8 out of 12 participants after the lipid infusion; however, the change in ceramide content did not reach statistical significance (Fig. 4a, p = 0.09). Variability in muscle DAG content was also observed following lipid infusion, such that there was no significant difference between the groups (Fig. 4a). There was sufficient muscle sample to measure the content of the different ceramide and DAG species by LC-MS/MS in seven participants. Of the 18 ceramide species measured, two were significantly downregulated following the lipid infusion: C14-Cer (29%, p < 0.05) and C18:1-Cer (21%, p < 0.05) (Fig. 4b). The content of C14:0/18:1-DAG increased by 34% (p < 0.05) while the content of C18:0/20:4-DAG decreased by 22% (p < 0.05) (Fig. 4c). Of the 15 alylcarnitines measured, the content of two long-chain acylcarnitines was significantly downregulated following lipid infusion: AC18:1n9 (35%, p < 0.05) and AC18:2n6 (33%, p < 0.05). Lipid infusion did not affect the content of short-, medium- or odd-chain acylcarnitines (Fig. 4d).
Fig. 4.
Effect of lipid infusion on total myocellular concentrations of ceramide and DAG (a) (n = 12) and of ceramide species (b), DAG species (c) (n = 7) and acylcarnitine species (d) (n=10). Results are means ± SE. *p < 0.05 and **p < 0.01 vs saline infusion
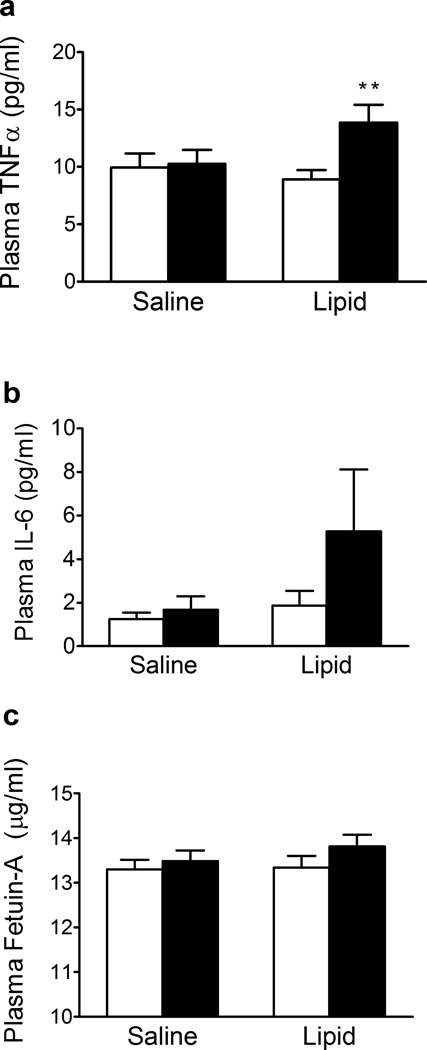
Effect of lipid infusion on plasma TNF-α, IL-6 and fetuin-A
Plasma TNF-α concentration was significantly increased following lipid but not saline infusion (Fig. 5a). Plasma IL-6 concentration was not affected by either saline or lipid infusion (Fig. 5b). We observed a trend for increased plasma concentrations of fetuin-A following lipid infusion (Fig. 5c, p = 0.08)
Fig. 5.
Effect of lipid infusion on plasma TNF-α (a) , IL-6 (b) and fetuin-A concentrations (c) before (white bars) and after (black bars) saline and lipid infusion. Results are means ± SE (n = 12). **p < 0.01 vs pre-infusion
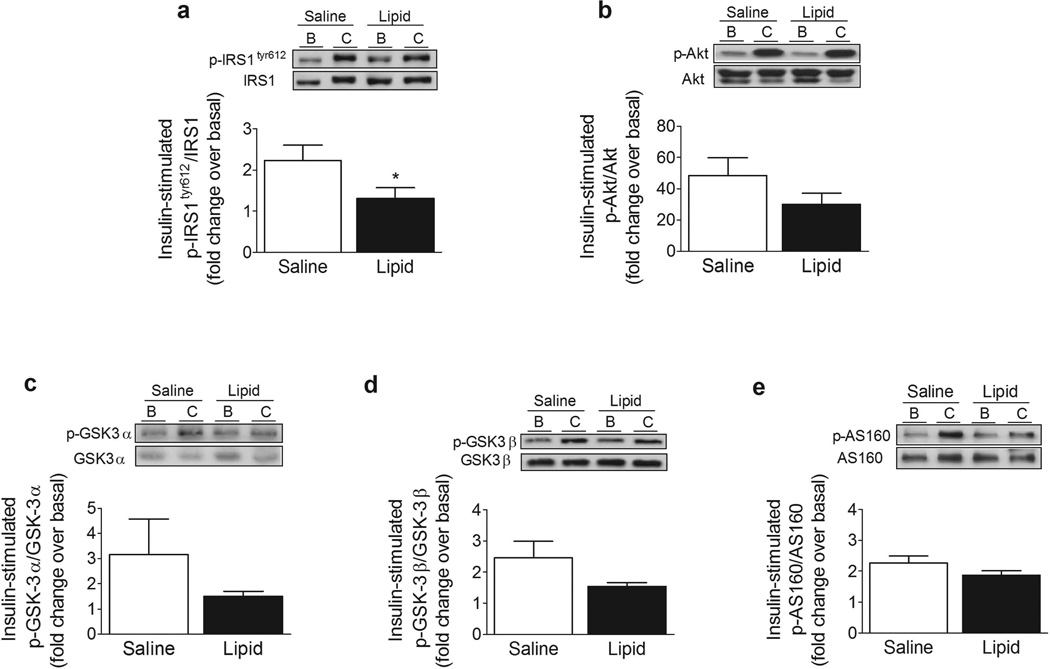
Effect of lipid infusion on the insulin receptor pathway
Considering that the lipid infusion induced an inflammatory response in muscle, we explored whether it affected the proximal and distal steps of the insulin signalling cascade. Serine phosphorylation of IRS-1 impairs insulin signalling by reducing tyrosine phosphorylation of IRS-1 by the insulin receptor β subunit. Both JNK and IKKβ [29] phosphorylate IRS-1 at Ser312 (Ser307 in rodent IRS-1) and this is thought to be a key mechanism by which NEFA and cytokines impair insulin signalling. Phosphorylation of IRS-1 at Ser1101 also impairs phosphorylation of IRS-1 at Tyr612 and downstream insulin signalling at the level of Akt and AS160 [7]. In the present study, lipid infusion caused a 70% reduction in insulin-stimulated phosphorylation of IRS-1 at Tyr612 (Fig. 6a, p < 0.05). In contrast to this suppressive effect on IRS-1, lipid infusion had no significant effect on phosphorylation of Akt (Fig. 6b) or its downstream target glycogen synthase kinase-3 (GSK-3)α/β (Fig. 6c, d). In addition, we observed no effect of lipid infusion on phosphorylation of AS160 (Fig. 6e), a key distal insulin signalling protein important for insulin-stimulated glucose transport.
Fig. 6.
Effect of lipid infusion on phospho-IRS-1tyr612 (a), phospho-Akt (b), phospho-GSK3α (c), phospho-GSK3β (d) and phospho-AS160 (e). Data are expressed as a ratio of phosphorylated to total protein, in arbitrary units, as insulin-stimulated fold change over basal. Representative immunoblots are shown. Results are means ± SE (n = 9). *p < 0.05 vs saline infusion
Discussion
In vitro studies provide compelling evidence that activation of inflammatory signalling pathways play a key role in NEFA-induced insulin resistance in muscle [8, 21, 22]. Lipid infusion is a well-established model with which to study mechanisms underlying NEFA-induced insulin resistance in vivo [3]. However, few studies have investigated the effect of lipid infusion on inflammation in skeletal muscle in humans [4]. Moreover, recent studies have challenged the notion that lipid infusion impairs insulin signalling in muscle [15] and alternative hypotheses, such as substrate competition [15, 30] and impaired insulin-stimulated microvasculature recruitment, have been suggested [31]. In the present study, a prolonged increase in plasma NEFA reduced whole-body glucose disposal in association with increased low-grade inflammation. Specifically, the lipid infusion caused an upregulation of genes and kinases within the TLR signalling network. Reduction in insulin-stimulated tyrosine phosphorylation of IRS-1 was also observed. Our findings suggest that increased flux through TLRs and downstream signalling pathways (NFκB and MAPK) may play an important role in the mechanism by which increased lipid supply induces insulin resistance in the muscle of insulin-resistant individuals. Some studies provide evidence that acute supraphysiological elevation in plasma NEFA concentration impairs insulin signalling at the level of IRS-1 (tyrosine phosphorylation and phosphatidylinositol 3-kinase [PI3K] association with IRS-1) [18, 19]. However, others have not observed such changes [13, 15]. Studies using a prolonged low-dose NEFA infusion without heparin, similar to that used in the present study, suggest a deleterious effect of lipid infusion on proximal steps of the insulin signalling cascade [32]. In accordance with these studies, we observed reduced insulin-stimulated IRS-1 tyrosine phosphorylation following low-dose lipid infusion. However, the lipid infusion had no effect on phosphorylation of Akt, GSK3α/β or AS160, more distal insulin signalling steps. Kruszynska et al previously reported a dissociation between the effects of lipid on insulin signalling at the level of IRS-1 and Akt in muscle of humans [18]. Kim et al also found that individuals with type 2 diabetes have impairments at the level of IRS-1-PI3K but not Akt [33]. Even though the cause for this dissociation between proximal and distal steps is not known, it is possible that distal signalling intermediaries (Akt, GSK3, AS160) are more sensitive than IRS-1 to the insulin dose (80–300 mU m−2 min) administered during the clamp in the present and previous [18, 33] studies. One could also speculate that partial activation of IRS-1 is sufficient for maximal Akt stimulation. Moreover, changes at the level of IRS-1 tyrosine phosphorylation, Akt, GSK3 and AS160 phosphorylation, detected in whole cell lysates, might not reflect changes within specific membrane compartments, which is an appreciated limitation of these experiments [34].
A major goal of the present study was to identify whether inflammatory responses and consequent impairments in insulin signalling, observed in cultured cells in vitro upon stimulation with NEFA, also occur during lipid-induced insulin resistance in humans. To the best of our knowledge, the present study is the first to investigate the effects of a lipid infusion on measures of MAPK signalling in humans. We observed a twofold increase in ERK phosphorylation as well as a trend for increased p38 and JNK phosphorylation following lipid infusion. In addition, there was an increase in the expression of MAPK8, the gene encoding for JNK1 protein, and MAP4K4, the gene encoding the kinase which regulates JNK1. Moreover, the expression of MAP2K3, the gene encoding for the protein which phosphorylates p38, was increased following lipid infusion. Increased basal activity/phosphorylation of JNK [12], ERK [10] and p38 [11] has been observed in skeletal muscle of insulin-resistant individuals. Studies in cells suggest a negative effect of these MAPKs on insulin signalling at the level of IRS-1 phosphorylation on serine residues [7]. Our observation that lipid infusion increased signalling through the MAPK pathway provides a potential mechanism for the impaired IRS-1 phosphorylation on tyrosine residues and reduced insulin sensitivity.
Studies in cultured cells and rodents have shown that NEFA increase signalling through the NFκB pathway [22, 23]. These studies mimic low-grade inflammatory conditions observed in muscle of individuals with obesity and type 2 diabetes [9, 25]. In the present study, lipid infusion increased the expression of CHUK, the gene encoding IKKα, part of the IKK enzyme complex which phosphorylates the IκB proteins, marking them for degradation. Accordingly, we observed a trend for reduced IκBα content together with increased expression of REL and RELA, genes encoding members of the NFκB family of transcription factors. Previous studies in rodents have reported reduced IκBα content in muscle following lipid infusion [35]. It is noteworthy that increased signalling through the NFκB pathway has been demonstrated in mononuclear cells following lipid infusion in humans [36]. Here we demonstrate that lipid infusion increases the muscle expression of genes encoding for proteins within the NFκB pathway that affect insulin action and glucose metabolism. Accordingly, there was a significant increase in circulating TNF-α following lipid infusion. Saturated fatty acids acutely stimulate TLR4 in monocytes/macrophages in vitro [37]. Thus, the increase in plasma TNF–α concentration suggests that Intralipid may have stimulated TLR4 in monocytes/macrophages, and possibly adipocytes, leading to an increase in the synthesis and release of cytokines into the circulation. This could be an additional mechanism by which the lipid infusion promoted an inflammatory state in muscle (increase in gene expression of inflammatory mediators) and impaired insulin action.
Two prevailing hypotheses explain how lipid oversupply upregulates inflammatory pathways and induce insulin resistance in muscle. Proponents of the lipotoxicity theory suggest that lipidderived metabolites (e.g. DAGs, ceramides) activate protein kinase Cs, MAPKs and IKK-NFκB, which impair insulin action at the level of IRS-1 and Akt [4, 38]. An alternative hypothesis considers NEFAs as ligands of TLR4/2, which stimulate downstream signalling through the NFκB/MAPK pathways [9, 21]. Although the present study does not conclusively prove both cause and effect, our data offers support for the latter mechanism. First, in contrast to previous studies that used lipid doses higher than the dose used in the present study [4, 14], total content of DAG and ceramide did not increase with Intralipid. Only one DAG (C18:0/20:4) and none of the ceramides increased after lipid infusion. Moreover, two ceramides (C14-Cer and C18:1-Cer) and one DAG (C14:0/18:1-DAG) were reduced following lipid infusion. Overall, these data suggest that accumulation of DAGs and/or ceramides is not a mechanism by which Intralipid induced insulin resistance in the present study, albeit the subcellular localisation of these lipid metabolites (not assessed in this study) may influence how they affect insulin action [39]. In addition to subcellular localisation, it will also be important to assess in future studies whether C18:0/20:4-DAG has a more potent effect in impairing insulin action than other DAG species. In addition to measuring DAG and ceramide content, we investigated the effect of lipid infusion on muscle acylcarnitine abundance. After transportation into the cell by fatty acid transporters, NEFA are activated by esterification to CoA. Subsequently, carnitine palmitoyltransferase 1 exchanges the CoA moiety for carnitine. The resulting acylcarnitine is transported across the inner mitochondrial membrane into the mitochondrion. It has been hypothesised that during excessive fuel (i.e. lipid) supply there is inefficient long-chain NEFA β oxidation, due in part to a relatively low tricarboxylic acid cycle capacity [40]. Presumably, this would lead to the accumulation of acylcarnitine molecules, which have been implicated in insulin resistance [40, 41]. Contrary to this hypothesis, long-chain acylcarnitine content was reduced following lipid infusion, and there was change in medium-, short- or odd-chain acylcarnitine content. Even though the cause of this paradoxical response is unknown (also observed with some DAGs and ceramides), it is probable that an adaptive response to increase lipid metabolite clearance is in play, such that the increased lipid supply acts to enhance lipid catabolism as part of a protective and compensatory negative feedback mechanism.
A recent study identified fetuin-A as an essential adaptor protein for the NEFA-mediated activation of TLR4 in mice [42]. In vitro, saturated lipids increased hepatic production of fetuin-A, which impairs insulin action through an NFκB-mediated mechanism [43]. Moreover, fetuin-A level was recently shown to correlate with insulin resistance in patients with type 2 diabetes [44]. In the present study, we observed a trend for increased plasma fetuin-A concentration following lipid infusion, raising the possibility that the observed inflammation/insulin resistance may be secondary to lipid-generated fetuin-A [43]. Future studies will be needed to clarify the role of fetuin-A on the NEFA-mediated activation of TLR4 in humans.
We have previously demonstrated increased TLR4 gene expression and signalling in the muscle of patients with obesity and type 2 diabetes [9]. Increased expression of TLRs also has been demonstrated by others in rodents [5, 45, 46] and humans [47–49]. Even though the precise fate of the lipids infused during the study cannot be known unless studies with labelled tracers were performed, our findings suggest that increases in TLR4 expression/signalling are acquired defects, secondary to excess NEFA supply to muscle. In the present study, gene expression of TLR4, but not other TLRs, was negatively correlated (r = −0.54, p = 0.02) with muscle insulin sensitivity (M value). The relevance of increased expression of TLR3 and TLR5 in muscle following lipid infusion is not clear since these TLRs have not been studied in the context of insulin resistance in humans. In rodents, TLR3 deficiency protects against diet-induced insulin resistance [50]. Future studies will be needed to establish whether there is a genetic (inherited) basis underlying augmented TLR expression in insulin-resistant muscle.
In summary, increases in TLR expression and flux though TLR-driven pathways (NFκB and MAPK), characteristic of insulin-resistant individuals, are reproduced by a sustained, mild elevation of plasma NEFA in normal-glucose-tolerant individuals. Strategies aimed at inhibiting flux through TLR signalling could be useful for the prevention and treatment of insulin-resistance disorders.
Supplementary Material
Acknowledgements
We thank all the study participants and their families for their time and participation.
Funding
This work was supported by grants from the National Institutes of Health (NIH) (RO1-DK80157 and RO1-DK089229) and the American Diabetes Association to NM. This grant also was supported by a University of Texas Health Science Center at San Antonio Clinical and Translational Science Award (TR000149) and an NIH National Service Research Award, Parent F32 (1F32DK095565-01A1) to SH. This project also was supported by the National Center for Research Resources and the Office of the Director of the NIH (C06 RR018823) to MUSC. Research was supported in part by the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (MUSC) (P30 CA138313) and the Lipidomics Core in the South Carolina Lipidomics and Pathobiology COBRE, Department Biochemistry, MUSC (P20 RR017677).
Abbreviations
- DAG
Diacylglycerol
- ERK
Extracellular signal-regulated kinase
- ESI-MS/MS
Electrospray tandem mass spectrometry
- GSK-3
Glycogen synthase kinase-3
- HOMA-IR
HOMA of insulin resistance
- IκBα
Inhibitor of kappa Bα
- IKK
Inhibitor of κB kinase
- JNK
c-Jun N-terminal kinase
- LC-MS/MS
High-performance liquid chromatography/mass spectrometry
- M
Insulin-stimulated glucose metabolism
- MAPK
Mitogen-activated kinase
- NFκB
Nuclear factor κB
- PI3K
Phosphatidylinositol 3-kinase
- TLR
Toll-like receptor
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SEH, HL, AA, YC, JGG, LA, JD and NM provided substantial contributions to the conception and design of this study and to the acquisition of data or analysis and interpretation of data. All authors were involved in drafting of the article or revising it critically for important intellectual content and had final approval of the version to be published.
References
- 1.Miles JM, Wooldridge D, Grellner WJ, et al. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes. 2003;52:675–681. doi: 10.2337/diabetes.52.3.675. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4649–4655. doi: 10.1210/jc.2004-0224. [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242. doi: 10.2337/diab.44.10.1239. [DOI] [PubMed] [Google Scholar]
- 4.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 7.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyna SM, Ghosh S, Tantiwong P, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouzakri K, Roques M, Gual P, et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 11.Koistinen HA, Chibalin AV, Zierath JR. Aberrant p38 mitogen-activated protein kinase signalling in skeletal muscle from type 2 diabetic patients. Diabetologia. 2003;46:1324–1328. doi: 10.1007/s00125-003-1196-3. [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54:2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- 13.Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D. Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme. AJ Clin Endocrinol Metab. 2007;92:3967–3972. doi: 10.1210/jc.2007-1104. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann OP, Dahl DB, Brechtel K, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 15.Hoeg LD, Sjoberg KA, Jeppesen J, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60:64–73. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vistisen B, Hellgren LI, Vadset T, et al. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol. 2008;158:61–68. doi: 10.1530/EJE-07-0493. [DOI] [PubMed] [Google Scholar]
- 17.Serlie MJ, Allick G, Groener JE, et al. Chronic treatment with pioglitazone does not protect obese patients with diabetes mellitus type II from free fatty acid-induced insulin resistance. J Clin Endocrinol Metab. 2007;92:166–171. doi: 10.1210/jc.2006-1518. [DOI] [PubMed] [Google Scholar]
- 18.Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab. 2002;87:226–234. doi: 10.1210/jcem.87.1.8187. [DOI] [PubMed] [Google Scholar]
- 19.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belfort R, Mandarino L, Kashyap S, et al. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005;54:1640–1648. doi: 10.2337/diabetes.54.6.1640. [DOI] [PubMed] [Google Scholar]
- 21.Radin MS, Sinha S, Bhatt BA, Dedousis N, O’Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336–346. doi: 10.1007/s00125-007-0861-3. [DOI] [PubMed] [Google Scholar]
- 22.Hussey SE, Liang H, Costford SR, et al. TAK-242, a small molecule inhibitor of toll-like receptor-4 signaling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci Rep. 2012;33:37–47. doi: 10.1042/BSR20120098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha S, Perdomo G, Brown NF, O’Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–41301. doi: 10.1074/jbc.M406514200. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Tantiwong P, Shanmugasundaram K, Monroy A, et al. NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab. 2010;299:E794–E801. doi: 10.1152/ajpendo.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 27.Dube JJ, Bhatt BA, Dedousis N, Bonen A, O’Doherty RM. Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2007;293:R642–R650. doi: 10.1152/ajpregu.00133.2007. [DOI] [PubMed] [Google Scholar]
- 28.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) Adv Exp Med Biol. 2010;688:46–59. doi: 10.1007/978-1-4419-6741-1_3. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 30.Hoy AJ, Brandon AE, Turner N, et al. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am J Physiol Endocrinol Metab. 2009;297:E67–E75. doi: 10.1152/ajpendo.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 33.Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulindependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3- kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue G, Cheatham B, Emkey R, Kahn CR. Dynamics of insulin signaling in 3T3- L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J Biol Chem. 1998;273:11548–11555. doi: 10.1074/jbc.273.19.11548. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt BA, Dube JJ, Dedousis N, Reider JA, O’Doherty RM. Diet-induced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol. 2006;290:R233–R240. doi: 10.1152/ajpregu.00097.2005. [DOI] [PubMed] [Google Scholar]
- 36.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 37.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 2012;55:1140–1150. doi: 10.1007/s00125-011-2419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 43.Dasgupta S, Bhattacharya S, Biswas A, Majumdar SS, Mukhopadhyay S, Ray S. NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem J. 2010;429:451–462. doi: 10.1042/BJ20100330. [DOI] [PubMed] [Google Scholar]
- 44.Jung CH, Kim BY, Kim CH, Kang SK, Jung SH, Mok JO. Associations of serum fetuin-A levels with insulin resistance and vascular complications in patients with type 2 diabetes. Diab Vasc Dis Res. 2013;10:459–467. doi: 10.1177/1479164113490766. [DOI] [PubMed] [Google Scholar]
- 45.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 46.Ladefoged M, Buschard K, Hansen AM. Increased expression of toll-like receptor 4 and inflammatory cytokines, interleukin-6 in particular, in islets from a mouse model of obesity and type 2 diabetes. APMIS. 2012;121:531–538. doi: 10.1111/apm.12018. [DOI] [PubMed] [Google Scholar]
- 47.Ahmad R, Al-Mass A, Atizado V, et al. Elevated expression of the toll like receptors 2 and 4 in obese individuals: its significance for obesity-induced inflammation. J Inflamm (Lond) 2012;9:48. doi: 10.1186/1476-9255-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 50.Wu LH, Huang CC, Adhikarakunnathu S, et al. Loss of toll-like receptor 3 function improves glucose tolerance and reduces liver steatosis in obese mice. Metabolism. 2012;61:1633–1645. doi: 10.1016/j.metabol.2012.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.