Abstract
Objectives
To test the effects of a modified Hospital Elder Life Program (mHELP) on frailty.
Design
Matched and unmatched analyses of data from a before-and-after study.
Setting
Hospital, inpatient.
Participants
Participants aged 65 years and older ((N = 189) undergoing elective, major abdominal surgery at a medical center in Taiwan.
Intervention
The mHELP included three nursing interventions: early mobilization, oral and nutritional assistance, and orienting communication.
Measurements
Frailty rate and transitions between frailty states from hospital discharge to 3 months after discharge, using Fried's phenotype criteria categorized as: non-frail (0 or 1 criteria present), pre-frail (2 or 3 criteria present), and frail (4 or 5 criteria present).
Results
Among matched pairs, participants who received the mHELP interventions were significantly less likely to be frail at discharge (19.2% vs. 65.4% for controls; adjusted OR = .10, 95% CI = .02 to .39) than their matched controls. Transitions to states of greater frailty during hospitalization were more common for participants in the control group. At 3 months post-discharge, participants who received the mHELP intervention during hospitalization were less likely to be frail than the matched controls (17.3% versus 23.1%; adjusted OR = .73; 95% CI = .21 to 2.56), although this difference did not achieve statistical significance.
Conclusion
The mHELP intervention is effective in reducing frailty by hospital discharge but the benefit is diminished by 3 months post-discharge. Thus, the mHELP provides a useful approach to manage in-hospital frailty for older patients undergoing major abdominal surgery.
Keywords: frailty, aged, intervention studies, surgery, geriatric syndromes
Introdction
Frailty, increasingly recognized as an overarching geriatric syndrome,1 is defined as a state of decreased reserve against stressors as a result of cumulative decline across multiple physiological systems.2 For older patients, frailty is highly prevalent and strongly associated with poor outcomes.3 A recent review concluded that although the prevalence rates of frailty were less than 10% in community-dwelling older adults, the rates among older hospitalized patients, particularly surgical patients, were much higher.4 Indeed, 42% of patients undergoing cardiac surgery and 50% of patients undergoing non-cardiac surgery were frail,4 highlighting the vulnerability of older surgical populations.
The impact of frailty is substantial as frailty is associated with higher rates of postoperative complications, longer length of stay, and higher rates of institutionalization.5 Despite the recognized importance of frailty, no effective treatment or intervention program has yet been designed to reduce frailty rates and alter transitions between frailty states.6
We adapted the successful Hospital Elder Life Program7-9 to focus on components that address the shared risk factors of cognitive, functional, and nutritional status and to enhance its feasibility and scalability in a surgical setting in Taiwan.10 The modification was based on the theory that many geriatric syndromes, including frailty, might be managed at once since many conditions “share” underlying risk factors.1,11-12
The overall aim of this before-and after intervention study was to evaluate the modified Hospital Elder Life Program (mHELP) comprising three nursing interventions (mobilization, oral and nutritional assistance, and orienting communication) for older Taiwanese patients undergoing common elective abdominal surgical procedures. Previously, we had reported its effects on reducing older surgical patients' functional decline and delirium rates by hospital discharge.13 Given that the mHELP intervention targets several geriatric syndromes (e.g., cognitive and functional status), we hypothesized that this mHELP intervention would affect frailty. We also hypothesized that older patients undergoing surgery, in particular abdominal, would have the greatest need for improved inpatient care. This is because older surgical patients are not only at higher risk of becoming frail,4 but also because their inpatient care is poor.14 Moreover, older patients undergoing major elective abdominal surgery are primarily for resection of malignancy and free from frailty is essential to reduce treatment toxicity and improve survival.15 The aim of the current study was to compare the rate of frailty and transitions between frailty states in older abdominal surgery patients receiving mHELP and usual care (participants enrolled before mHELP implemented) at hospital discharge and at 3 months after discharge.
For the present study, we addressed the imbalance in frailty at baseline in the intervention and usual care groups by a retrospective, individual 1:1 matching16 to ensure that participants in the two groups were comparable with respect to frailty state, age, and comorbidity burden. Then, matched and unmatched analyses were performed to examine the immediate and short-term effects on frailty both upon hospital discharge and 3 months post-discharge.
Methods
Setting and Participants
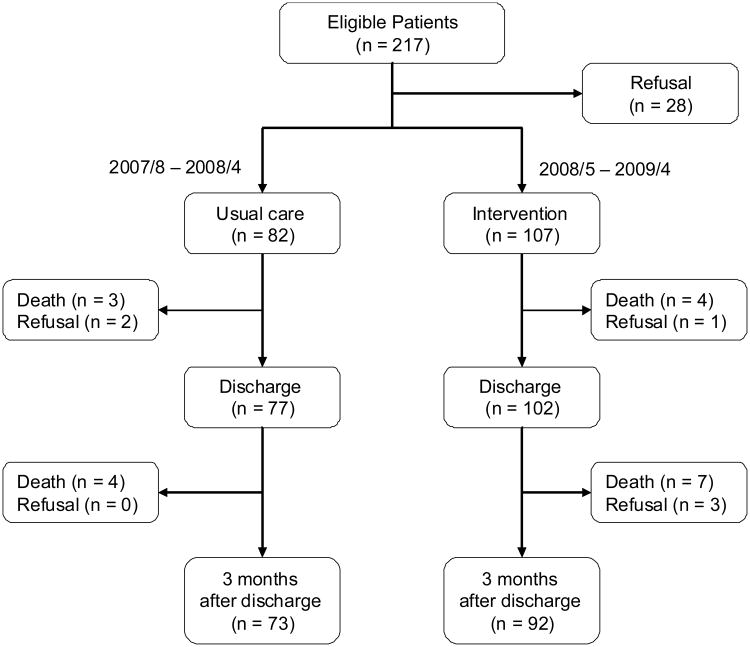
A before-and-after intervention study was conducted to test the effects of a mHELP intervention for older Taiwanese patients scheduled for major elective abdominal surgery. The Research Ethics Review Committee of the medical center approved the study. All participants provided written informed consent for study participation. Participants who completed the study are the focus of this report. The study flowchart detailing the attrition from the two groups is shown in Figure 1. The original sample included consecutive older patients (> 65 years old, N=189) admitted to the 36-bed gastrointestinal ward of an urban medical center in Taiwan and scheduled for elective abdominal surgery with an expected length of stay > 6 days, as described in detail previously.13 Patients admitted from August 2007 to April 2008 served as the usual care group (n = 82) and patients admitted from May 2008 to April 2009 comprised the intervention group (n = 107).
Figure 1. The Study Flowchart.
Although, contamination was not a factor in the before-after study design, frailty at baseline differed significantly between the two groups (Table 1). Participants were matched individually (1:1) post-hoc to ensure that the two groups were as comparable as possible at baseline.16,17 We therefore retrospectively matched participants from the mHELP to the usual care group. Participants were included only if they had completed both discharge and 3-month evaluations and the match was performed to by a research associate who was blinded to the outcome to avoid bias. The research associate identified one matched control for each mHELP participant using baseline characteristics reported to be associated with postsurgical frailty in the following order: frailty state (exact match on non-frail, pre-frail, and frail states), Charlson co-morbidity (exact match on 0, 1, 2, and 2+ comorbid diagnoses), and age (±5 years). The study sample included 52 matched pairs, with the additional 75 and 61 participants included only in the unmatched analyses for discharge and 3 months post-discharge, respectively.
Table 1. Sample Demographic and Medical Characteristics.
| Matched Pairs a | All Participants b | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | mHELP n = 52 | Usual Care n = 52 | p | mHELP n = 107 | Usual Care n = 82 | p |
| Demographic characteristic | ||||||
| Age (years), mean (SD) | 72.8 (5.5) | 72.2 (5.6) | - c | 73.3 (6.2) | 72.8 (5.6) | 0.43 |
| Female, n (%) | 24 (46.2) | 24 (46.2) | 1.00 | 47 (43.9) | 35 (42.7) | 0.98 |
| Education (years), mean (SD) | 8.4 (4.9) | 6.8 (5.4) | 0.12 | 8.4 (4.7) | 6.5 (5.7) | 0.02 |
| Medical characteristic | ||||||
| Principal Diagnosis, n (%) | ||||||
| Gastric cancer | 13 (25.0) | 22 (42.3) | 0.11 | 35 (32.7) | 30 (36.6) | 0.69 |
| Periampullary cancer | 18 (34.6) | 8 (15.4) | 0.06 | 31 (29.0) | 12 (14.6) | 0.03 |
| Distal pancreatic cancer | 6 (11.5) | 4 (7.7) | 0.68 | 8 (7.5) | 6 (7.3) | 1.00 |
| Other d | 15 (28.8) | 18 (34.6) | 0.66 | 33 (32.8) | 34 (41.5) | 0.22 |
| Malignancy, n (%) | 40 (76.9) | 39 (75.0) | 1.00 | 87 (81.3) | 61 (74.4) | 0.32 |
| Charlson index, mean (SD) | 1.6 (1.5) | 1.6 (1.7) | - c | 1.5 (1.5) | 2.2 (2.2) | 0.03 |
| Type of surgical procedure | ||||||
| Open, n (%) | 40 (76.9) | 45 (86.5) | 0.36 | 78 (72.9) | 71 (86.6) | 0.04 |
| Laparoscopic, n (%) | 5 (9.6) | 0 (0) | NA e | 10 (9.3) | 0 (0) | 0.01 |
| Laparoscopic-assisted, n (%) | 7 (13.5) | 7 (13.5) | 1.00 | 19 (17.8) | 11 (13.4) | 0.54 |
| Duration of surgery (min), mean (SD) | 226.2 (94.0) | 203.0 (70.0) | 0.15 | 226.8 (91.1) | 199.0 (68.7) | 0.02 |
| Length of hospital stay (days), mean (SD) | 17.6 (12.7) | 18.1 (13.6) | 0.85 | 17.3 (11.0) | 20.5 (18.2) | 0.17 |
| Baseline Frailty, n (%) | ||||||
| Frail (4 or 5 criteria) | 6 (11.5) | 6 (11.5) | - c | 8 (7.5) | 18 (22.0) | <.01 |
| Pre-Frail (2 or 3 criteria) | 44 (84.6) | 44 (84.6) | - c | 67 (62.6) | 58 (70.7) | 0.31 |
| Non-Frail (0 or 1 criteria) | 2 (3.8) | 2 (3.8) | - c | 32 (29.9) | 6 (7.3) | <.01 |
Notes.
Differences in participants' characteristics between matched pairs were evaluated using the McNemar test for binary variables and the paired t-test for continuous variables.
Differences in participants' characteristics between all samples were evaluated using chi-square test or Fisher's exact test for binary variables and the t-test or Wilcoxon rank-sum test for continuous variables.
The association between the matching variable and exposure (intervention) is broken by cohort matching, so the hypothesis testing is not meaningful.28
Diagnoses included gastrointestinal stromal tumor (GIST), appendiceal cancer, ileal tumor, ischemia bowel, colon tumor, and common bile duct adenocarcinoma.
The McNemar test was unable to obtain the p-value due to zero cell count.
Modified HELP Interventions
The mHELP comprised three mHELP interventions (early mobilization, oral and nutritional assistance, and orientating communication). Participants in the intervention group received mHELP in addition to usual care as soon as they arrived on the surgical inpatient ward. Using standardized mHELP manuals, a registered nurse with over 2 years of experience in medical-surgical nursing was trained as the HELP nurse. This 2-month on-site training included review of manuals and weekly individual mentorship. This same HELP nurse, who was blinded to the study hypotheses and did not serve as an outcome assessor, provided all three modified HELP interventions three times daily during the study. In the mobilization intervention, this HELP nurse assisted patients in completing physical activities, including range-of-motion exercises in bed, sitting up, riding a stationary bicycle by hand/foot, standing, or ambulating, according to their capacity. While carrying out activities, the HELP nurse deliberately engaged participants in orienting communication, e.g., recalling/discussing issues that interested them, such as events on the operative day, thus reinforcing orienting content. Daily oral care (tooth brushing and range-of-motion exercises for lips/tongue/jaw) and diet education for post-surgical intake were also provided. Approximately 30-45 minutes per day were added to patient care for each participant as a result of the mHELP protocol.10 The intervention ended upon hospital discharge and no extra care was provided during the 3-month follow-up period. The majority of participants (54%) received approximately 7 days (range = 4 to 20) of the mHELP interventions.
Usual Care
Usual care consisted of standard hospital care provided by physicians and nurses, similar to a hospital in the US. Referral to a dietician or physical therapist was on an as-needed basis, but the same group of nurses and attending physicians provided care to patients in both the intervention and usual care groups.
Study and Outcome Data
Data on study variables and outcomes were collected by two nurses trained as outcome assessors. All participants from both intervention and usual care groups were evaluated in face-to-face encounters upon admission, before discharge, and 3 months after discharge. The two nurses worked side-by-side and did not participate in any of the interventions; however, blinding to the intervention and usual care status was not possible due to the before-after study design. To ensure reliability and validity of measures, the two outcome assessors achieved an inter-rater reliability of .95 or higher before study start-up with the first author (CCC) on key measures (i.e., the Enforced Social Dependency Scale,18 Geriatric Depression Scale,19 and grip strength to define frailty criteria) and underwent performance checks every 3 months to avoid deviation from the measurement protocol.
Independent variables included participants' demographic (age, gender, and education) and medical (principal diagnosis, comorbidities, type and duration of surgery, length of stay, and frailty at admission baseline) characteristics. Frailty was the outcome variable at two time points: discharge and 3 months post-discharge. Data on demographic and medical characteristics including principal diagnosis, malignancy, comorbidities, type and duration of surgery, and length of stay were obtained from the medical record. Comorbidities were based on the Charlson comorbidity index, in which patients' weighted comorbidities are summed to obtain a score; higher scores indicate higher mortality risk.20
Frailty (yes/no) was determined by meeting 4 out of 5 Fried's criteria: 2,5 Shrinking (weight loss), weakness, exhaustion, low activity, and slow walking speed. Specifically, shrinking (yes/no) was defined as measured weight loss > 5% compared to the previous time point. For example, the shrinking criterion was met if body weight at admission was 5% less than at 3 months before admission, body weight at hospital discharge was 5% less than at admission, or body weight at 3 months was 5% less than at discharge, as measured by a portable digital scale (Tanita Corp., Japan) met this criterion. For weakness (yes/no), the criterion was met when grip strength, assessed as the average of two readings by a digital, handheld dynamometer (GRIP-D, T.K.K 5401; Takei Scientific Instruments Co., Ltd), was less than or equal to the sex-and body mass index-specific cutoff points provided by Fried et al. 2 The criterion for exhaustion (yes/no) was met by answering “no” to the question of one item question “Do you feel full of energy?” on the short version of Geriatric Depression Scale.19 Low activity level (yes/no), based on item 7 of the Enforced Social Dependence Scale (ESDS),18 was categorized when a participant was coded as “yes” by independent assessors if a participant had one of the following activity levels: “restricted activity-some activities characterizing work role can not longer performed”; “works half as much time as before or less”; or “no activity-major activities defining role are no longer being performed.” Slow walking speed (yes/no), based on item 3 of the ESDS, was coded as “yes” if participants had one of the following conditions: “walks with help of equipment or other person”; “does not walk”; or “unable to take any steps.” Furthermore, since frailty state (number of frailty criteria) was shown to have a dose-response relationship with outcomes,21,22 transitions between frailty states were also evaluated. Frailty state was coded as suggested: 5 non-frail (0 or 1 criteria present), pre-frail (2 or 3 criteria present), and frail (4 or 5 criteria present).
Statistical Analysis
Data were double-entered to ensure accuracy and analyzed using SAS version 9.2, with the significance level set at p <.05. Analyses were done on a per-protocol basis. Differences in participants' characteristics between matched pairs were evaluated using the McNemar test for binary variables and the paired t-test for continuous variables. To evaluate the intervention effect, pairwise differences in frailty (yes/no) were tested by conditional logistic regression, using usual care as the reference group, to estimate the odds ratio and 95% confidence interval.23 Baseline cohort differences for matched pairs that may confound the relationship between frailty and intervention effects were adjusted in conditional logistic regression. We purposefully selected the p-value cutoff of 0.2 to control for as many potential variables as possible and to avoid the possibility of a more restrictive level (e.g., p < 0.05) failing to identify variables known to be important.24 Logistic regression, using the entire sample (N=189), was used to test the robustness of the matched results. In this confirmatory unmatched analysis, baseline cohort differences for the entire sample (p < 0.2) were adjusted as control variables. Furthermore, transitions between frailty states in matched pairs are visually presented. The x-axis represents the frailty states at admission (baseline) for both groups and the y-axis shows the rates of transitions to each frailty state by hospital discharge/or 3-month follow-up. The Bhapkar test, a generalization of the McNemar test used for a square table with more than two rows/columns, was used to test for between-groups statistical significance in transitions between frailty states.25
Results
Sample characteristics at hospital admission are reported in Table 1. Baseline characteristics are presented for subset of matched pairs (n = 104) and for all participants enrolled in the study (unmatched, N = 189). Among the matched pairs, participants receiving mHELP and usual care were comparable in age, gender, malignant diagnosis, Charlson co-morbidity level, certain type of surgical procedure, length of hospital stay, and baseline frailty level. However, an increase in laparoscopic procedures occurred during the study period, from 0% in the usual care group to 9.8% (n = 5) in the intervention group, and the most common diagnosis, for the usual care group was gastric cancer, but the mHELP group had more periampullary cancer (34.6% versus 15.4% in the usual care group, p = .06).
Frailty rate at hospital discharge
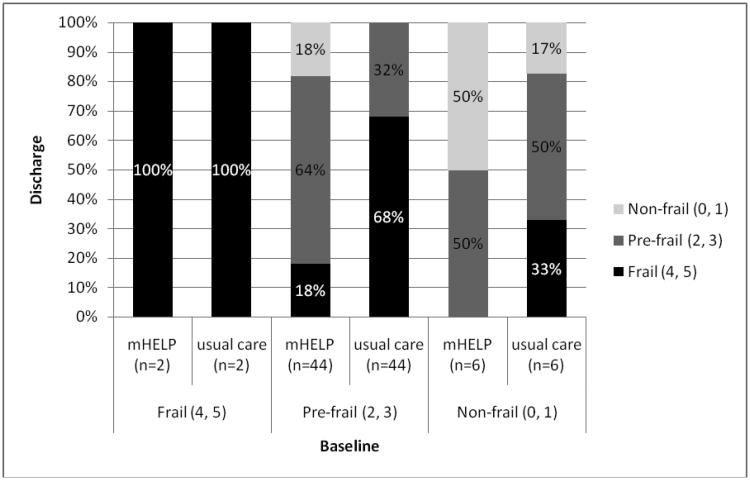
For the matched pairs, 19.2% of the participants receiving mHELP were frail by hospital discharge, versus 65.4% of the usual care controls (p ≤ .001, Table 2). Conditional regression analysis revealed that participants receiving mHELP were 90% less likely to be frail by hospital discharge than those receiving usual care (adjusted odds ratio (OR) =0.10, 95% confidence interval (CI) =0.02 to 0.39), after adjusting for education years, gastric cancer diagnosis (yes/no), periampullary cancer diagnosis (yes/no), duration of surgery (minutes), and type of surgical procedure (open/laparoscopic & laparoscopic-assisted). Results are similar in the unmatched analysis (adjusted OR=0.05, 95% CI=0.02 to 0.15; Table 2), adjusted for education years, periampullary cancer diagnosis, duration of surgery, type of surgical procedure, Charlson comorbidities, length of hospital stay (days), and baseline frailty levels (frail/pre-frail/non-frail). Transitions between frailty states also differed significantly with more transitions to states of lesser frailty occurring in the mHELP group (Figure 2a). Namely, for patients who were pre-frail upon admission, 68% of participants receiving usual care advanced to a frail state with 32% remaining in a pre-frail state, while only 18% of participants receiving mHELP advanced to frail states, 64% remained at pre-frail, and the remaining 18% improved to non-frail, a less frail state (p < .001).
Table 2. Rate of Frailty in Participants Receiving mHELP and Usual Care.
| Type of Analysis | n/N (%) | P-value | Multivariate Odds Ratio (95% confidence interval) | |
|---|---|---|---|---|
|
| ||||
| mHELP | Usual care | |||
| Matched Pairs (52 pairs) a | ||||
| Frail c at discharge | 10/52 (19.2) | 34/52 (65.4) | .001 | 0.10 (0.02-0.39) |
| Frail c at 3mo after discharge | 9/52 (17.3) | 12/52 (23.1) | .62 | 0.73 (0.21-2.56) |
| Unmatched (N=189) b | ||||
| Frail c at discharge | 15/102 (14.7) | 52/77 (67.5) | <.001 | 0.05 (0.02-0.15) |
| Frail c at 3mo after discharge | 14/92(15.2) | 22/73 (30.1) | .72 | 0.85 (0.34-2.09) |
Notes.
Conditional logistic regression models adjusted for education (years), periampullary cancer (yes/no), gastric cancer (yes/no), duration of surgery (min), and type of surgical procedure (open vs. laparoscopic/laparoscopic-assisted) were used to obtain odds ratio (OR), 95% confidence interval (CI), and p-value (p).
Logistic regression models were adjusted for education, periampullary cancer, Charlson comorbidities, type of surgical procedure, duration of surgery, and length of hospital stay (days), and baseline frailty (frail/pre-frail/non-frail).
Frailty (yes/no) was determined by meeting four of five Fried's criteria
Figure 2a. Transitions between frailty States (from admission to discharge) for 52 matched pairs.
Notes. The x-axis represents the frailty states at admission (baseline) for both groups, and the y-axis shows the rates of transitions to each frailty state by hospital discharge
Frailty rate 3 months post-discharge
For the matched pairs, with no intervention provided after discharge, 3 months after hospital discharge, 17.3% of participants receiving mHELP were frail versus 23.1% of usual care controls (p = .62, Table 2). Conditional logistic regression, adjusted for education, periampullary cancer, gastric cancer, duration of surgery, and type of surgical procedure, indicated that participants receiving mHELP were 27% less likely to be frail at 3 months after hospital discharge than those receiving usual care (adjusted OR =0.73, 95% CI =0.21 to 2.56; Table 2). Results are similar in the unmatched analysis (adjusted OR=0.85, 95% CI=0.34 to 2.09; Table 2). Results for transition of frailty states show a positive trend for participants receiving mHELP to transition to states of lesser frailty (Figure 2b), particularly among participants who were in the pre-frail or non-frail states at baseline. More participants (21%) who received mHELP during hospitalization improved to not-frail at 3 months from pre-frail at baseline, compared to only 9% of usual care controls, although this difference did not achieve statistical significance (p = .28). However, the study lacked adequate power at the 3-month follow-up (power was only 5% for matched pairs and 80% for the unmatched sample with two-sided testing and α level set at .05).24 These results thus support a positive trend for the benefit of the mHELP intervention for frailty at 3 months after hospital discharge, particularly for patients who were less frail before surgery.
Figure 2b. Transitions between frailty States (from Admission to 3 months after Discharge) for 52 matched pairs.
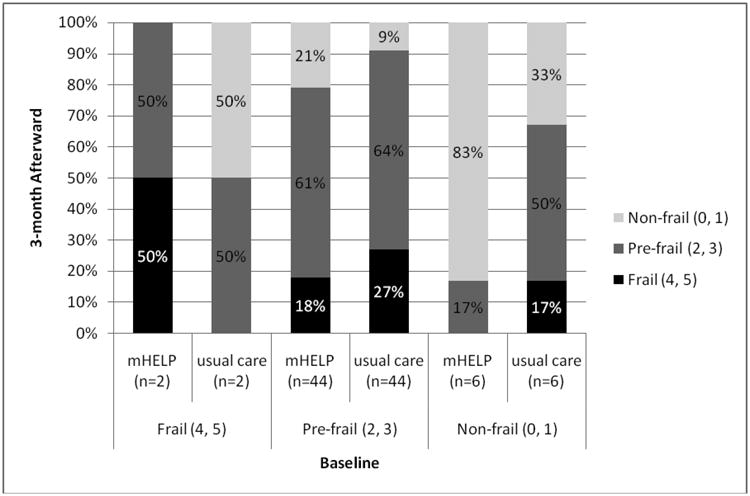
Notes. The x-axis represents the frailty states at admission (baseline) for both groups, and the y-axis shows the rates of transitions to each frailty state by 3-month follow-up
Discussion
The mHELP was associated with an approximately 90% reduction in risk of frailty at hospital discharge compared with usual care in patients undergoing common elective abdominal surgical procedures in Taiwan. At 3 months post-discharge, participants receiving mHELP were 27% less likely to be frail, but the difference was not statistically significant. Furthermore, participants who received mHELP were more likely to transition to a lesser state of frailty (e.g. from pre-frail to non-frail) by hospital discharge. Specifically, patients who did not meet criteria for frailty at hospital admission (i.e., were non-frail or pre-frail) were more likely to benefit from the mHELP. For example, 18% of pre-frail participants who received mHELP were improved to a non-frail state, while none of the participants in the usual care group transitioned from pre-frail to non-frail. Similarly, with the support of the mHELP intervention, 50% of non-frail participants remained non-frail and none advanced to the frail state, versus only 17% of controls who remained non-frail while 33% advanced to the frail state. These results suggest that the mHELP impacts on frailty and the transition between frailty states and that mHELP is particularly beneficial in pre-frail states. Future work is needed to confirm these preliminary findings.
Two points need to be emphasized in light of the current findings. First, consistent with the existing literature,4,5 the prevalence of frailty is high among older Taiwanese patients undergoing surgical procedures, and effective hospital-based intervention programs are greatly needed. For all participants receiving usual care, at hospital discharge, frailty developed in 67.5% of older patients undergoing elective, major abdominal surgery. This rate remained high at 30.1% by 3 months post-discharge. Thus, the mHELP comprising three nursing interventions (targeting cognition, function, and nutrition) provided a feasible approach to reduce frailty rates and reverse transitions to a more severe frailty state.
Our study suggests that for older patients recovering from abdominal surgery, early mobilization, orienting communication, and dietary education along with daily oral care including tooth brushing and orofacial range-of-motion exercise reduced shrinking (weight loss), weakness, and eventual frailty states during the course of hospitalization. Oral and nutrition assistance was developed as one of three shared-risk-factor-interventions in the mHELP. Nutrition is a long-standing problem in surgical patients and likely contributes to poor outcomes, but few studies have offered specific intervention strategies. Beyond the complex nature of malnutrition, many factors pose barriers to addressing the issue including the lack of simple, measurable interventions, particularly in the busy hospital setting.26 These findings demonstrate that mHELP not only alleviates nutritional problems but also ameliorates eventual frailty in the hospital setting. Confirmation is needed in future trials with larger samples.
Second, the positive findings of this study support the notion that “shared risk factors” are not only applicable to the common geriatric syndromes of delirium and functional decline,13 but also to the resulting frailty occurring by the time of hospital discharge. As such, the mHELP addressing three shared risk factors has high potential to interrupt self-sustaining pathways (i.e., shared risk factors resulting in geriatric syndromes, which lead to frailty) resulting in poor outcomes, and providing an opportunity to advance the quality of care for the rapidly growing elderly surgical population.
Unfortunately, 3 months after discharge, the difference between groups in frailty status was not statistically significant. Given that no intervention was provided after patients were discharged from the hospital, it is not surprising that benefits were time-limited. We did not collect data on time to initiation of adjuvant chemotherapy, so we cannot exclude the possibility that these participants receiving mHELP were less frail upon hospital discharge and were more likely to start adjuvant therapy sooner, and therefore, were likely be more frail by 3 months after discharge (due to side effects of chemotherapy). Given that most patients were either frail or pre-frail by hospital discharge and many were scheduled for adjuvant therapy to improve survival, the urgent need for transitional care is indicated. A boosting program or ideas such as “HELP at home” might provide a way to sustain the momentum, particularly for those at high risk of increasing frailty status.
The current study has important limitations. First, we used five study variables approaching Fried's frailty phenotype. However, an important omission is that we did not directly measure gait speed; instead we used a self-reported measure of walking limitation. Thus, we acknowledge the possibility that the study's main outcome represents a different construct than Fried's frailty construct and caution is needed in interpreting our results. Second, we used historical controls in this before-after study. Participants were enrolled in two different years, and we did not use methods for achieving balanced allocation (e.g., randomization, prospective matching). Thus, the study groups had baseline imbalances. In addition, temporal trends in clinical practice, such as increased use of laparoscopic procedures and shorter lengths of hospital stay may have accounted for the superior outcomes, rather than the intervention per se. We adjusted for all identified differences in post-hoc analyses. All cohort differences were carefully adjusted in unmatched and matched analyses where participants from the intervention and usual care groups were matched on several baseline characteristics, including frailty level, age, and comorbid disease burden. While these methods suggest that the findings are robust, we cannot rule out temporal trends or unmeasured confounders contributing to our findings. Thus, these results must be interpreted with caution and replicated in the setting of a randomized clinical trial. Third, analyses were performed on a per-protocol basis; thus, inferences were conditional on patient survival and attrition. The impact of this effect was limited by 1) a low attrition rate of 5.3% (70% were deaths) at hospital discharge and 12.7% (75% were deaths) at 3 months follow-up; and 2) comparable attrition rates between groups (14% for intervention vs. 11% for controls at 3 months). Moreover, the study was underpowered to detect the intervention effect at the 3 months after discharge, potentially explaining the non-statistically significant finding at the 3-month follow-up. Larger samples are recommended for future trials. Nevertheless, the positive findings of this pilot trial justify future randomized controlled trials to evaluate the effect of a mHELP on frailty, in order to determine whether frailty can be reduced or reversed for older surgical patients.
Conclusion
The modified HELP comprising three nursing interventions significantly reduces frailty and transitions to higher frailty states by hospital discharge for older patients undergoing common abdominal surgical procedures. While encouraging trends were demonstrated at 3 months post-discharge, the improvement did not achieve statistical significance, which was not surprising given that no extra care was provided after patients were discharged from the hospital. These findings highlight the importance of improving important patient outcomes by focusing on basic areas of care and targeting care to address shared risk factors rather than single conditions.
Acknowledgments
This study was supported in part by the Taiwan National Science Council Grant #95-2314B002-188-MY3, by a career development grant from the National Health Research Institute (Grant #NHRI-EX-9820PC), and by the Hospital Elder Life Program. Dr. Saczynski was supported in part by funding from the National Institute on Aging (K01AG33643) and the National Heart Lung and Blood Institute (U01HL105268). Dr. Sharon Inouye holds the Milton and Shirley F. Levy Family Chair.
Sponsor's Role: None of the funding agencies had any role in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflict of interest
Author Contributions: Study concept and design: Cheryl Chia-Hui Chen, Chiung-Nien Chen, I-Rue Lai, Sharon Inouye. Acquisition of subjects and data: Cheryl Chia-Hui Chen, Chiung-Nien Chen, I-Rue Lai. Analysis and interpretation of data: Cheryl Chia-Hui Chen, Guan-Hua Huang, Jane Saczynski, Sharon Inouye. Drafting or revising manuscript for important intellectual content: Cheryl Chia-Hui Chen, Chiung-Nien Chen, I-Rue Lai, Guan-Hua Huang, Jane Saczynski, Sharon Inouye. Approval of final version to be published: Cheryl Chia-Hui Chen, Chiung-Nien Chen, I-Rue Lai, Guan-Hua Huang, Jane Saczynski, Sharon Inouye.
References
- 1.Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Partridge JSL, Harari D, Dhesi JK. Frailty in the older surgical patient: A review. Age Ageing. 2012;41:142–147. doi: 10.1093/ageing/afr182. [DOI] [PubMed] [Google Scholar]
- 5.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Fairhall N, Langron C, Sherrington C, et al. Treating frailty-a practical guide. BMC Med. 2011;9:83–90. doi: 10.1186/1741-7015-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK, Bogardus ST, Jr, Baker DI, et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 9.SteelFisher GK, Martin LA, Dowal SL, et al. Sustaining Clinical Programs during Difficult Economic Times: A Case Series from the Hospital Elder Life Program. J Am Geriatr Soc. 2011;59:1873–1812. doi: 10.1111/j.1532-5415.2011.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CCH, Saczynski J, Inouye SK. The modified hospital elder life program: Adapting a complex intervention for feasibility and scalability in a surgical setting. J Gerontol Nurs. doi: 10.3928/00989134-20140110-01. in press. [DOI] [PubMed] [Google Scholar]
- 11.Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–1353. [PubMed] [Google Scholar]
- 12.Chen CCH, Dai YT, Yen CJ, et al. Shared risk factors for distinct geriatric syndromes in older Taiwanese inpatients. Nurs Res. 2010;59:340–347. doi: 10.1097/NNR.0b013e3181eb31f6. [DOI] [PubMed] [Google Scholar]
- 13.Chen CCH, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: Effects on abdominal surgery patients. J Am Coll Surg. 2011;213:245–252. doi: 10.1016/j.jamcollsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Parker SG, Conroy S. Poor inpatient care for older people. BMJ. 2011;342:d373. doi: 10.1136/bmj.d373. [DOI] [PubMed] [Google Scholar]
- 15.Kurian AA, Wang L, Grunkemeier G, et al. Defining the elderly undergoing major gastrointestinal resections. Ann Surg. 2013;258:483–489. doi: 10.1097/SLA.0b013e3182a196d8. [DOI] [PubMed] [Google Scholar]
- 16.Rothman K, Greenland S. Modern Epidemiology. 2nd. Philadelphia, PA: Lippincott, Williams and Wilkins; 1998. [Google Scholar]
- 17.Costanza MC. Matching. Prev Med. 1995;24:425–433. doi: 10.1006/pmed.1995.1069. [DOI] [PubMed] [Google Scholar]
- 18.Benoliel JQ, McCorkle R, Young K. Development of a social dependency scale. Res Nurs Health. 1980;3:3–10. doi: 10.1002/nur.4770030103. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression scale: A preliminary report. J Psychiatr Res. 1983;17:31–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 22.Kulminski AM, Ukraintseva SV, Kulminskaya IV, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: Lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gail MH, Lubin JH, Rubinstein LV. Likelihood calculations for matched case-control studies and survival studies with tied death times. Biometrika. 1980;68:703–707. [Google Scholar]
- 24.Micky J, Greenland S. A study of the impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 25.Bhapkar VP. A note on the equivalence of two test criteria for hypotheses in categorical data. J Am Statistical Assoc. 1966;61:228–235. [Google Scholar]
- 26.National Cancer Institute Power Program [on-line] [Accessed May 14, 2013]; Available at http://biometry.nci.nih.gov/cgi-bin/power2/option6.pl?sopt=2.
- 27.Starke J, Schneider H, Alteheld B, et al. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clin Nutr. 2011;30:194–201. doi: 10.1016/j.clnu.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Jewell N. Statistics for Epidemiology. Boca Raton, Florida: Chapman & Hall/CRC Press; 2004. [Google Scholar]