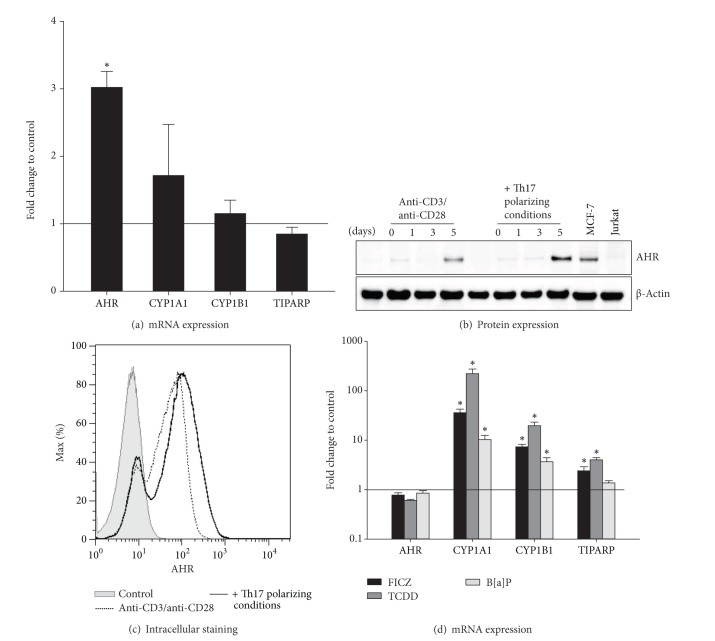
Figure 5.
The AHR is induced in CD4+ T cells and activated by FICZ, TCDD, and B[a]P. Human CD4+ T cells were cultured with anti-CD3/anti-CD28 in the presence or absence of Th17 polarizing conditions for 5 days. (a) The mRNA expression of the indicated genes was measured by real-time PCR. Data were normalized to the mean of the reference genes GUSB, PPIA, PGK1, and the mRNA expression at day 0 using the ΔΔCt-method. Fold changes in gene expression of CD4+ T cells with Th17 polarizing conditions compared to control (black line) are represented as mean and SEM. *represents significance, P < 0.05, Mann-Whitney U test, and n = 7. (b) The AHR protein expression (94 kDa) at days 1, 3, and 5 of culture was measured by Western Blot analysis. The protein expression of unstimulated CD4+ T cells was used as control. Beta-Actin (42 kDa) antibody was used as loading control. Cell lysates from MCF-7 cell line and Jurkat T cell line were used as AHR positive and negative control, respectively. One representative of 3 independent experiments is shown. (c) After restimulation with PMA, Ionomycin, and Monensin for 5 hours and fixation/permeabilization, the cells were stained for CD4 and AHR and analyzed by flow cytometry. Unstained cells were used as control n = 2. (d) The AHR ligands FICZ (100 nM), TCDD (5 nM), and B[a]P (100 nM) were added to CD4+ T cells cultured with Th17 polarizing cytokines. CD4+ T cells treated with anti-CD3/anti-CD28, Th17 polarizing conditions, and DMSO (0.05%) were used as control. Relative mRNA expression of the indicated genes was measured by real-time PCR and data were normalized to the mean of GUSB, PPIA, and PGK1 and to the mRNA expression at day 0 using the ΔΔCt-method. Data are shown as fold changes of mRNA expression in Th17 polarized and ligand treated CD4+ T cells compared to control (black line) and are represented as mean and SEM; *represents significance, P < 0.05, Mann-Whitney U test, and n = 7.