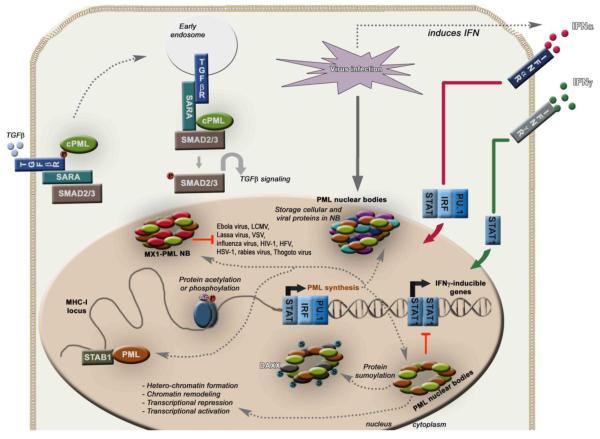
Figure 5. The role of TRIM19/PML in antiviral defense, signal transduction and induction by IFNs.
Mechanisms of TRIM19 up-regulation upon viral infection via IFN production. TRIM19 is an important effector of the IFN-mediated antiviral response. TRIM19 exerts its functions by interacting with other proteins including SP100 and DAXX to form nuclear bodies (NB). These protein complexes in NB are important in regulation of transcription, storage of proteins, sumoylation and antiviral functions. TRIM19 can regulate chromatin remodeling of the MHC-1 locus, and inhibit replication of viruses like influenza virus, Thogoto virus, herpes simplex virus-1 (HSV-1), Ebola virus, lymphocytic choriomeningitis virus (LCMV), Lassa virus, vesicular stomatitis virus (VSV), rabies virus, HIV-1, human foamy virus (HFV). TRIM19 also negatively regulates IFNγ signaling by inhibiting STAT1 transcriptional activity. The cytoplasmic isoform of TRIM19 (cPML) binds to the TGFβ receptor and serves as an adaptor molecule to recruit SARA and SMAD2/3 for TGFβ signaling.