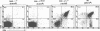Abstract
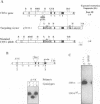
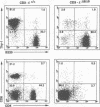
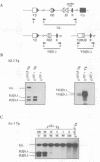
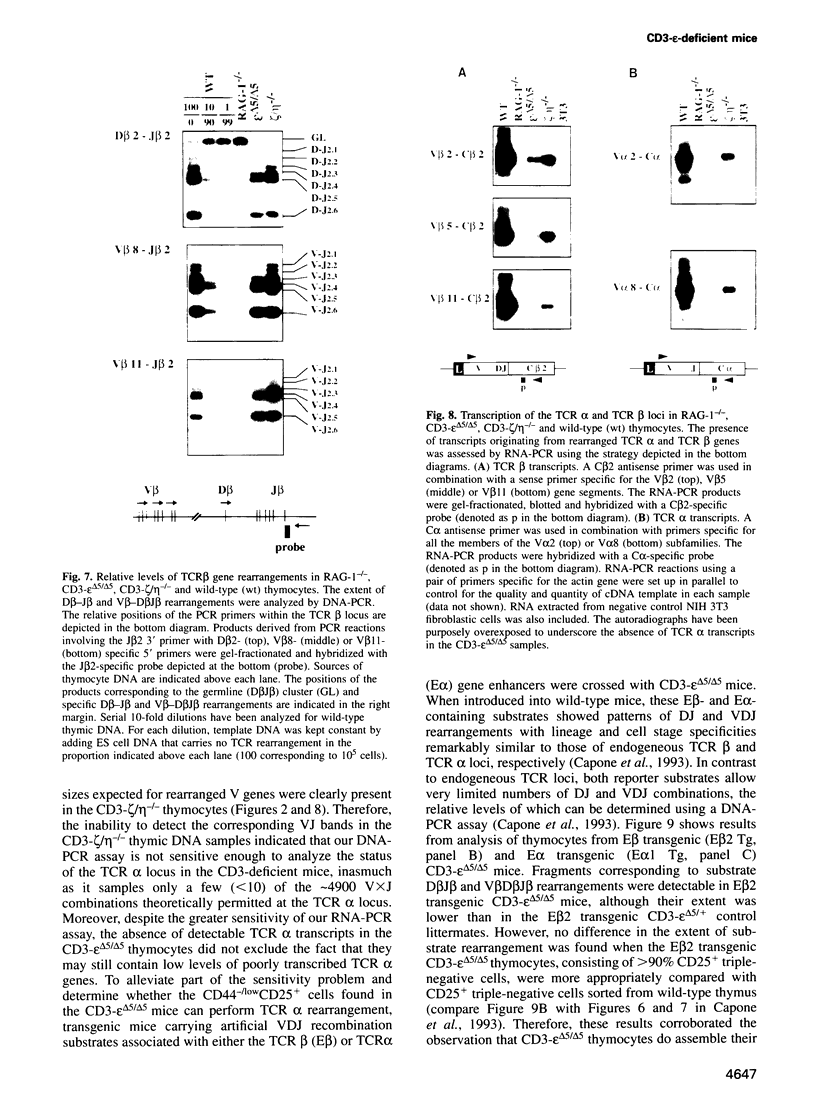
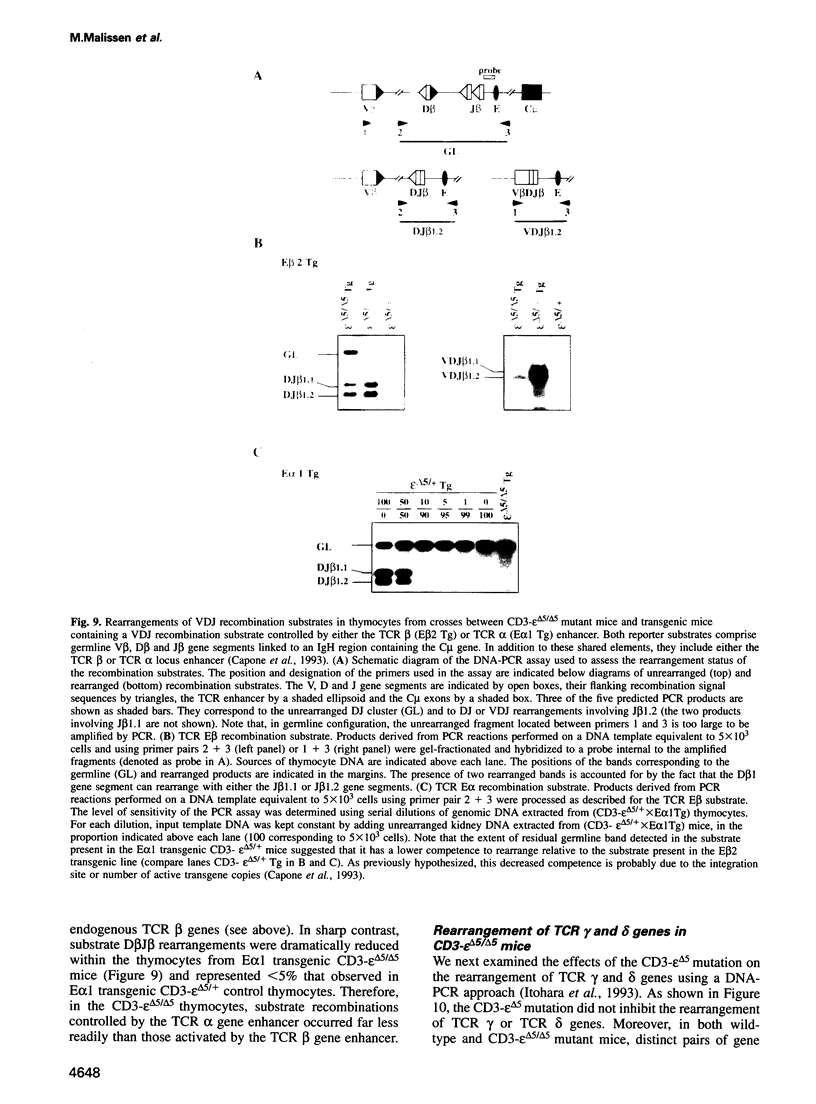
To determine which CD3 components are required for early T cell development, we generated mice with a targeted mutation of the CD3-epsilon gene and characterized their T cell populations relative to those found in CD3-zeta/eta-and recombinase activating gene (RAG)-deficient mice. In the absence of intact CD3-epsilon subunit, thymocytes do not progress beyond the CD44-/lowCD25+ triple-negative stage and appear to be arrested at the very same developmental control point as RAG-deficient thymocytes. In contrast, the disruption of the CD3-epsilon/eta gene does not totally abrogate the progression through this control point. CD3-epsilon-deficient thymocytes do rearrange their T cell receptor (TCR) beta gene segments and produce low levels of full-length TCR beta transcripts. Taken together, these results establish an essential role for the CD3-epsilon gene products during T cell development and further suggest that the CD3-epsilon polypeptides start to exert their function as part of a pre-TCR through which CD44-/lowCD25+ triple-negative cells monitor the occurrence of productive TCR beta gene rearrangements. Finally, the absence of intact CD3-epsilon polypeptides had no discernible effect on the completion of TCR gamma and TCR delta gene rearrangements, emphasizing that they are probably not subjected to the same epigenetic controls as those operating on the expression of TCR alpha and beta genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Abraham K. M., Nakayama T., Singer A., Perlmutter R. M. Inhibition of T-cell receptor beta-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992 Dec;11(13):4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artelt P., Grannemann R., Stocking C., Friel J., Bartsch J., Hauser H. The prokaryotic neomycin-resistance-encoding gene acts as a transcriptional silencer in eukaryotic cells. Gene. 1991 Mar 15;99(2):249–254. doi: 10.1016/0378-1119(91)90134-w. [DOI] [PubMed] [Google Scholar]
- Berget S. M. Exon recognition in vertebrate splicing. J Biol Chem. 1995 Feb 10;270(6):2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- Capone M., Watrin F., Fernex C., Horvat B., Krippl B., Wu L., Scollay R., Ferrier P. TCR beta and TCR alpha gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO J. 1993 Nov;12(11):4335–4346. doi: 10.1002/j.1460-2075.1993.tb06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Dunlap S., Saito H., Georgopoulos K., Wileman T., Terhorst C. Characterization and expression of the murine CD3-epsilon gene. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8623–8627. doi: 10.1073/pnas.85.22.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton T., Moore M., MacDonald H. R., Malissen B. Double-negative thymocyte subsets in CD3 zeta chain-deficient mice: absence of HSA+CD44-CD25- cells. Eur J Immunol. 1994 Aug;24(8):1903–1907. doi: 10.1002/eji.1830240828. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Reipert B. M., Dexter T. M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993 Sep 10;74(5):823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I., Kennedy J., Mombaerts P., Tonegawa S., Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8- thymocyte differentiation. J Immunol. 1994 May 15;152(10):4783–4792. [PubMed] [Google Scholar]
- Godfrey D. I., Kennedy J., Suda T., Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993 May 15;150(10):4244–4252. [PubMed] [Google Scholar]
- Godfrey D. I., Zlotnik A. Control points in early T-cell development. Immunol Today. 1993 Nov;14(11):547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Groettrup M., Baron A., Griffiths G., Palacios R., von Boehmer H. T cell receptor (TCR) beta chain homodimers on the surface of immature but not mature alpha, gamma, delta chain deficient T cell lines. EMBO J. 1992 Jul;11(7):2735–2745. doi: 10.1002/j.1460-2075.1992.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groettrup M., Ungewiss K., Azogui O., Palacios R., Owen M. J., Hayday A. C., von Boehmer H. A novel disulfide-linked heterodimer on pre-T cells consists of the T cell receptor beta chain and a 33 kd glycoprotein. Cell. 1993 Oct 22;75(2):283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A. R., Hooper M. L., Farr A., Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993 Feb 12;72(3):337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Vandeputte D., Tolkamp L., de Vries E., Borst J., Berns A. CD3 components at the surface of pro-T cells can mediate pre-T cell development in vivo. Eur J Immunol. 1994 Apr;24(4):934–939. doi: 10.1002/eji.1830240423. [DOI] [PubMed] [Google Scholar]
- Kisielow P., von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Köntgen F., Süss G., Stewart C., Steinmetz M., Bluethmann H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int Immunol. 1993 Aug;5(8):957–964. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Mattei M. G., Malissen B. The mouse CD3-gamma, -delta, and -epsilon genes reside within 50 kilobases on chromosome 9, whereas CD3-zeta maps to chromosome 1, band H. Immunogenetics. 1989;29(4):265–268. doi: 10.1007/BF00717911. [DOI] [PubMed] [Google Scholar]
- Levelt C. N., Carsetti R., Eichmann K. Regulation of thymocyte development through CD3. II. Expression of T cell receptor beta CD3 epsilon and maturation to the CD4+8+ stage are highly correlated in individual thymocytes. J Exp Med. 1993 Dec 1;178(6):1867–1875. doi: 10.1084/jem.178.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt C. N., Mombaerts P., Iglesias A., Tonegawa S., Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor beta-chain-deficient mutant mice by transmembrane signaling through CD3 epsilon. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S. D., Anderson S. J., Forbush K. A., Perlmutter R. M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993 Apr;12(4):1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Ueda R., She J., Sancho J., Wang B., Weddell G., Loring J., Kurahara C., Dudley E. C., Hayday A. Abnormal T cell development in CD3-zeta-/- mutant mice and identification of a novel T cell population in the intestine. EMBO J. 1993 Dec;12(12):4863–4875. doi: 10.1002/j.1460-2075.1993.tb06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Shores E. W., Johnson M. D., Tremblay M. L., Lee E. J., Grinberg A., Huang S. P., Singer A., Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993 Aug 13;261(5123):918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Rocha B., Trucy J., Vivier E., Boyer C., Köntgen F., Brun N., Mazza G., Spanopoulou E. T cell development in mice lacking the CD3-zeta/eta gene. EMBO J. 1993 Nov;12(11):4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Jouvin-Marche E., Cazenave P. A., Scollay R., Malissen B. Regulation of TCR alpha and beta gene allelic exclusion during T-cell development. Immunol Today. 1992 Aug;13(8):315–322. doi: 10.1016/0167-5699(92)90044-8. [DOI] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Manolios N., Kemp O., Li Z. G. The T cell antigen receptor alpha and beta chains interact via distinct regions with CD3 chains. Eur J Immunol. 1994 Jan;24(1):84–92. doi: 10.1002/eji.1830240114. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Sutherland L. C., Adra C. N., Leclair B., Rudnicki M. A., Jardine K. The mouse Pgk-1 gene promoter contains an upstream activator sequence. Nucleic Acids Res. 1991 Oct 25;19(20):5755–5761. doi: 10.1093/nar/19.20.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Anderson S. J., Perlmutter R. M., Mak T. W., Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994 Jul;1(4):261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Pearse M., Wu L., Egerton M., Wilson A., Shortman K., Scollay R. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt J. A., Kubo R. T., Saito T., Finkel T. H., Kathiresan S., Blank K. J., Hashimoto Y. Surface expression of a T cell receptor beta (TCR-beta) chain in the absence of TCR-alpha, -delta, and -gamma proteins. J Exp Med. 1991 Oct 1;174(4):775–783. doi: 10.1084/jem.174.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Karasuyama H., Haasner D., Grawunder U., Mårtensson I. L., Kudo A., Melchers F. Two pathways of B-lymphocyte development in mouse bone marrow and the roles of surrogate L chain in this development. Immunol Rev. 1994 Feb;137:185–201. doi: 10.1111/j.1600-065x.1994.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V., Chen D., Diamond R. A. Functional and phenotypic analysis of thymocytes in SCID mice. Evidence for functional response transitions before and after the SCID arrest point. J Immunol. 1993 Oct 1;151(7):3530–3546. [PubMed] [Google Scholar]
- Saint-Ruf C., Ungewiss K., Groettrup M., Bruno L., Fehling H. J., von Boehmer H. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994 Nov 18;266(5188):1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Alt F. W. CD3 epsilon-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2-/- mice in the absence of TCR beta chain expression. Int Immunol. 1994 Jul;6(7):995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Koyasu S., Nakayama K., Murphy K. M., Loh D. Y., Reinherz E. L., Alt F. W. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993 Feb 5;259(5096):822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Ma A., Cheng H. L., Alt F. W. CD3 epsilon and CD3 zeta cytoplasmic domains can independently generate signals for T cell development and function. Immunity. 1995 Apr;2(4):401–411. doi: 10.1016/1074-7613(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Shores E. W., Huang K., Tran T., Lee E., Grinberg A., Love P. E. Role of TCR zeta chain in T cell development and selection. Science. 1994 Nov 11;266(5187):1047–1050. doi: 10.1126/science.7526464. [DOI] [PubMed] [Google Scholar]
- Shores E. W., Sharrow S. O., Uppenkamp I., Singer A. T cell receptor-negative thymocytes from SCID mice can be induced to enter the CD4/CD8 differentiation pathway. Eur J Immunol. 1990 Jan;20(1):69–77. doi: 10.1002/eji.1830200111. [DOI] [PubMed] [Google Scholar]
- Spanopoulou E., Roman C. A., Corcoran L. M., Schlissel M. S., Silver D. P., Nemazee D., Nussenzweig M. C., Shinton S. A., Hardy R. R., Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 1994 May 1;8(9):1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- Takeda S., Zou Y. R., Bluethmann H., Kitamura D., Muller U., Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO J. 1993 Jun;12(6):2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Biron C., She J., Higgins K., Sunshine M. J., Lacy E., Lonberg N., Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Levelt C., Salio M., Zheng D., Sancho J., Liu C. P., She J., Huang M., Higgins K., Sunshine M. J. Over-expression of CD3 epsilon transgenes blocks T lymphocyte development. Int Immunol. 1995 Mar;7(3):435–448. doi: 10.1093/intimm/7.3.435. [DOI] [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Wiest D. L., Kearse K. P., Shores E. W., Singer A. Developmentally regulated expression of CD3 components independent of clonotypic T cell antigen receptor complexes on immature thymocytes. J Exp Med. 1994 Oct 1;180(4):1375–1382. doi: 10.1084/jem.180.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Kane L. P., Young J., Carson G. R., Terhorst C. Associations between subunit ectodomains promote T cell antigen receptor assembly and protect against degradation in the ER. J Cell Biol. 1993 Jul;122(1):67–78. doi: 10.1083/jcb.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Held W., MacDonald H. R. Two waves of recombinase gene expression in developing thymocytes. J Exp Med. 1994 Apr 1;179(4):1355–1360. doi: 10.1084/jem.179.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Scollay R., Egerton M., Pearse M., Spangrude G. J., Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 1991 Jan 3;349(6304):71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]