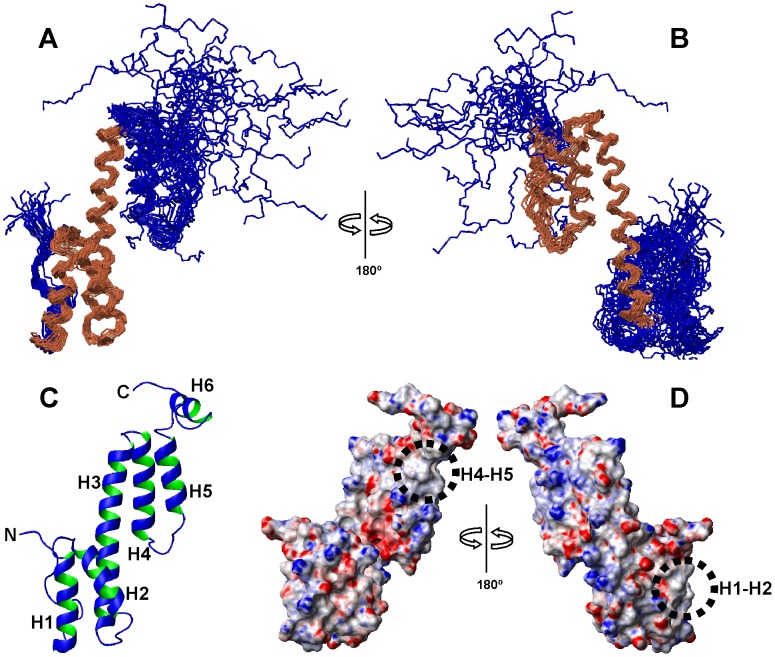
Figure 1. Solution structure of Ani s 5.
A, Backbone superposition of helices H1–H3 of the 20 lowest-energy conformers of Ani s 5 obtained by NMR. The superimposed helices are in gold and the rest of the protein is in blue. B, Backbone superposition of helices H3–H5 of the 20 lowest-energy conformers of Ani s 5 obtained by NMR. The superimposed helices are in gold and the rest of the protein in blue. The orientation of the ensemble is rotated 180° in A and B. C, Ribbon display of a representative conformer of the family showing the positions of the helical segments (H1 to H5) and one of the possible orientations of the C-terminal tail containing helix H6, with respect to the protein core. Helices H1 (15–27), H2 (33–47), H3 (51–77), H4 (82–96), H5 (103–113), H6 (118–125) and the N- and C-termini are labeled. D, Electrostatic surface of Ani s 5. Negative charges are in red, positive charges are in blue. Circles indicate the hydrophobic regions at the level of H1–H2 and H4–H5 helices. Left, the protein has the same orientation as in A. Right, 180° rotated view.