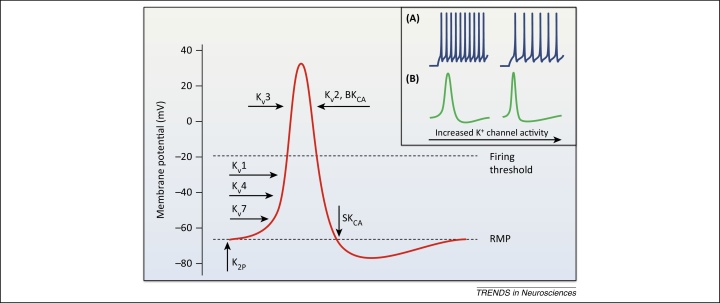
Figure 1.
Potassium channel activation during action potential (AP) firing in sensory neurons. A depiction of the sequential engagement of different K+ channels during neuronal activity, and typical effects of K+ channel opening on AP waveform and frequency (inset). The resting membrane potential (RMP) is primarily stabilized by two-pore K+ (K2P) channels and Kv7 background conductance, whereas KATP channels may also contribute in large neurons [95]. Basal excitability is also influenced by the opening of low-threshold Kv1 and Kv4 channels which filter out small depolarizations and therefore control the number of triggered APs. Kv4 channels are normally inactivated at RMP and require prior hyperpolarization (achieved during AP generation) to remove this steady-state inactivation. Once activated, however, Kv4 and other A-type channels may modulate firing threshold as well as repetitive spiking rate owing to their very fast kinetics [inset (A)] [47]. Following suprathreshold stimulation and initiation of an AP, high-threshold Kv3 channels open to limit AP duration and ensure quick recovery of voltage-gated Na+ channels from inactivation [inset (B)]. Kv2 channels are also high-threshold but with much slower activation and inactivation kinetics; they mainly contribute to the repolarizing/after-hyperpolarizing phases and are hence important for regulating interspike interval and conduction fidelity during sustained stimulation [inset (B)] [38]. Upon neuronal activity, Ca2+-activated K+ channels are engaged during repolarization (BKCA) and after-hyperpolarization (SKCA) to provide feedback inhibition at nerve terminals by restricting AP duration and thus neurotransmitter release [(inset (B)]. It is emphasized that this schematic is a simplified representation of most prominent K+ channel contributions to AP firing, based on in vitro assessment of recombinant counterparts. In vivo, however, the oligomeric composition, association with auxiliary proteins, post-translational modifications, and regulation by intracellular messengers can yield divergent biophysical properties. K+ channel opening will also have concurrent effects on the function of other ion channels, for instance by affecting their inactivation. For this reason the combined effect on firing behavior in a physiological context is often hard to predict. Abbreviations: BKCA, big conductance and SKCA, small conductance Ca2+-activated K+ channels.