Abstract
Objective: In the past, the authors performed a comprehensive literature review to identify all randomized controlled trials assessing the impact of early tracheostomy on severe brain injury outcomes. The search produced only two trials, one by Sugerman and another by Bouderka. Subjects and methods: The current authors initiated an Institutional Review Board-approved severe brain injury randomized trial to evaluate the impact of early tracheostomy on ventilator-associated pneumonia rates, intensive care unit (ICU)/ventilator days, and hospital mortality. Current study results were compared with the other randomized trials and a meta-analysis was performed. Results: Early tracheostomy pneumonia rates were Sugerman-48.6%, Bouderka-58.1%, and current study-46.7%. No early tracheostomy pneumonia rates were Sugerman-53.1%, Bouderka-61.3%, and current study-44.4%. Pneumonia rate meta-analysis showed no difference for early tracheostomy and no early tracheostomy (OR 0.89; p = 0.71). Early tracheostomy ICU/ventilator days were Sugerman-16 ± 5.9, Bouderka-14.5 ± 7.3, and current study-14.1 ± 5.7. No early tracheostomy ICU/ventilator days were Sugerman-19 ± 11.3, Bouderka-17.5 ± 10.6, and current study-17 ± 5.4. ICU/ventilator day meta-analysis showed 2.9 fewer days with early tracheostomy (p = 0.02). Early tracheostomy mortality rates were Sugerman-14.3%, Bouderka-38.7%, and current study-0%. No early tracheostomy mortality rates were Sugerman-3.2%, Bouderka-22.6%, and current study-0%. Randomized trial mortality rate meta-analysis showed a higher rate for early tracheostomy (OR 2.68; p = 0.05). Because the randomized trials were small, a literature assessment was undertaken to find all retrospective studies describing the association of early tracheostomy on severe brain injury hospital mortality. The review produced five retrospective studies, with a total of 3,356 patients. Retrospective study mortality rate meta-analysis demonstrated a larger mortality for early tracheostomy (OR 1.97; p < 0.0001). Conclusion: For severe brain injury, analyses indicate that ventilator-associated pneumonia rates are not decreased with early tracheostomy. Further, this study implies that mechanical ventilation is reduced with early tracheostomy. Both the randomized trial and retrospective meta-analysis indicate that risk for hospital death increases with early tracheostomy. Findings imply that early tracheostomy for severe brain injury is not a prudent routine policy.
Keywords: Head injuries, tracheostomy, hospital mortality
Introduction
In 2006, the first and fourth authors published a systematic literature review and meta-analysis assessing outcomes for trauma patients undergoing early tracheostomy [1]. That review indicated there were no survival and no ventilator associated pneumonia (VAP) benefits with early tracheostomy. However, meta-analysis showed a trend (p = 0.06) for fewer ventilator/intensive care unit (ICU) days in the only two published randomized controlled trials of severe brain injured patients. These studies were published by Sugerman in 1997 [2] and Bouderka in 2004 [3]. Based on those results, the authors initiated an IRB-approved randomized controlled trial to evaluate the impact of early tracheostomy in patients with severe brain injury.
This manuscript describes the findings of our randomized controlled trial and compares them with the other two severe brain injury randomized controlled trials. Study entry criteria, randomization process, timing of tracheostomy, and method for diagnosing VAP for each study is described. Severity of illness, VAP rates, ventilator/ICU days, and mortality rates are given for the early and no early tracheostomy groups in each study. Finally, the studies are combined and a meta-analysis is performed to determine the effect of early tracheostomy on the VAP rate, ventilator/ICU duration, and mortality rate.
Materials and methods
St. Elizabeth Health Center (SEHC) randomized trial
Ethics Statement: The SEHC Institutional Review Board approved the study and the written informed consent. Informed consent was signed by the patient’s representative, following an extensive discussion about the trial.
SEHC patient study entry criteria included blunt trauma, admission Glasgow Coma Score (GCS) ≤ 8, intracranial hemorrhage seen on brain computed tomography scan, and age 18-65 years old. Patients were excluded if they had cardiac arrest, near-brain death, pre-existing coagulopathy, or severe obesity.
The SEHC randomization process occurred on hospital day three. After consent was obtained, the patient was assigned to the early or no early tracheostomy group from a set of cards placed in random order, using computer-generated random numbers. For a few patients where families voiced a strong opinion, early tracheostomy or no early tracheostomy was according to their request. Early tracheostomy was performed on post-injury day 3-5. The no early tracheostomy group was assessed at post-injury day 10-14 and underwent tracheostomy, if endotracheal tube extubation was not imminent.
Each day, randomized patients underwent a daily assessment for suspected VAP. Daily, patients were assessed for a chest x-ray infiltrate (new, persistent, or progressive), abnormal WBC count (< 4,000 or > 11,000 cells per microliter or immature neutrophils ≥ 10%), abnormal temperature (≥ 101.2 or ≤ 97.6 degrees Fahrenheit), and P/F O2 ≤ 240. VAP was suspected when the following two criteria were present: (1) chest x-ray infiltrate or P/F O2 ≤ 240 and (2) purulent tracheal secretions, abnormal WBC count, or abnormal temperature. When suspicion for VAP was present, a bronchoalveolar lavage was performed and empiric antimicrobial therapy was initiated. The patient was considered to have VAP, if the lavage result demonstrated bacteria with ≥ 104 colony forming units per mL. VAP rates came from a dedicated, prospective data collection process.
Ventilator days for the SEHC study came from a dedicated, prospective data collection process. Hospital mortality for the SEHC study was prospectively documented. Due to a slow accrual rate of appropriate patients, the SEHC study was terminated.
Historic randomized trials
Historic randomized trial results were included to compare with SEHC outcomes. Results from the Sugerman and Bouderka studies are provided, because they are the only two published randomized controlled trials addressing early tracheostomy in patients with severe brain injury. The Sugerman study included patients with major head trauma (GCS ≤ 8) who had undergone three days of mechanical ventilation. If an additional seven days of ventilation were anticipated, consent was obtained. The Bouderka study consisted of patients with isolated severe head injury (admission GCS ≤ 8) and cerebral contusion on computed tomography scan. GCS score ≤ 8 on the fifth day without any sedation was also required.
The Sugerman study randomized patients on hospital day three, after consent was obtained. Patients were randomized from a set of cards placed in random order, using computer-generated random numbers. The Bouderka study patients were randomized on hospital day five. Details of the randomization process were not described.
Timing of early tracheostomy for the Sugerman patients was 3-5 days post-injury and for the Bouderka patients was day 5 or 6 post-injury. If necessary, late tracheostomy was performed on day 10-14 in the Sugerman study. The timing of late tracheostomy was not stipulated in the Bouderka publication.
Severity of illness data were obtained from the Sugerman and Bouderka publications. VAP rates were obtained from the Sugerman and Bouderka publications. ICU days were available in the Sugerman article and ventilator days were given in the Bouderka publication. Hos- pital mortality rates were obtained from the Sugerman and Bouderka publications.
VAP in the Sugerman study was based on positive tracheal aspirate culture results in patients with clinically suspected VAP. Clinical suspicion included an altered white blood cell count, pyrexia, and a new or progressive chest x-ray infiltrate. The Bouderka study used VAP criteria published by the Centers for Disease Control in 1988.
Aggregate assessment of randomized trials
VAP rates for the three randomized studies were compared for the early and no early tracheostomy groups. A meta-analysis was performed to determine the effect of early tracheostomy on VAP. Ventilator/ICU days for the three studies were compared for the early and no early tracheostomy groups. Effect size of the ICU or ventilator days was determined using meta-analysis to determine the impact of early tracheostomy. In the same manner as described for VAP rates, mortality rates were compared and a meta-analysis was performed to determine the effect of early tracheostomy.
Retrospective studies
Because the randomized trial meta-analysis P-value for mortality was equivocal, an extensive PubMed search was performed to find all retrospective study publications describing hospital mortality outcomes in severe brain injured patients, according to the time of tracheostomy. Hospital mortality rates, according to time of tracheostomy, were described for each retrospective study and a meta-analysis was performed.
Statistics
The SAS System for Windows, release 9.2 (SAS Institute Inc., Cary, NC, USA) was used to perform SEHC study statistical analysis. Continuous variables are expressed as mean and standard deviation. Statistical relationships were assessed using the following techniques: a) t-test for comparison of interval continuous data between two groups; b) Wilcoxon rank-sum test for comparison of ordinal-rank continuous data between two groups; and c) Fisher’s exact test to assess 2x2 contingency tables. We considered p < 0.05 to represent statistical significance. Data results and P-values from the literature reviews are those presented in the publications. Data reported as standard error of the mean was converted to standard deviation. A fixed-effects meta-analysis with Review Manager 4.2 for Windows (Oxford, The Cochrane Collaboration, 2003) was used to assess combined study data. Meta-analysis was used to assess relative risk for hospital mortality and VAP rates. Meta-analysis was also used to assess the standardized mean difference, mean of early tracheostomy group minus mean of no early tracheostomy group divided by standard deviation, for ventilator/ICU days.
Results
SEHC randomized trial
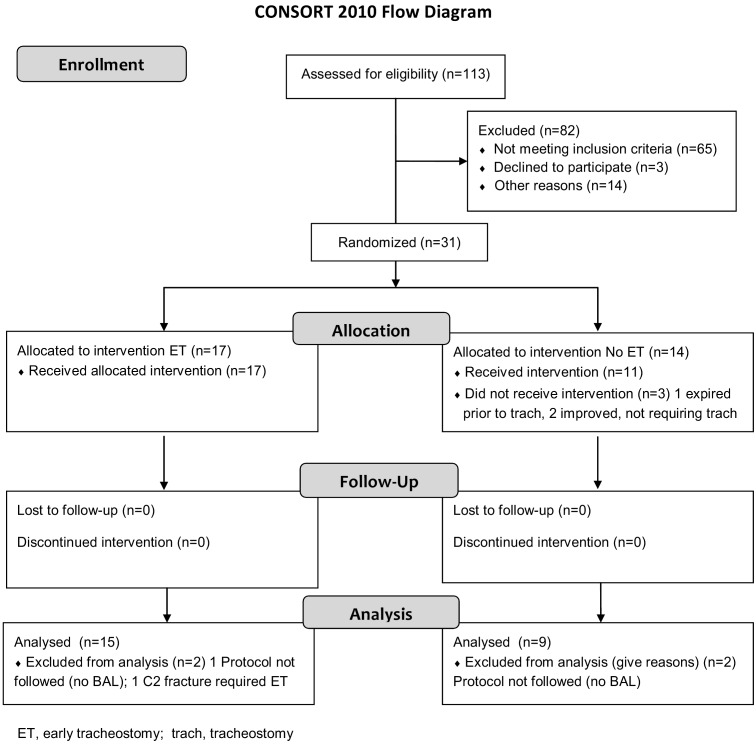
SEHC initial patient assessment, group allocation, and follow-up are described in the Consolidated Standards of Reporting Trials (CONSORT) flow chart (Figure 1). Mechanisms of injury were vehicular (automobile, motorcycle, or pedestrian struck) 20 (83.3%), assault 1 (4.2%), fall 1 (4.2%), and struck by falling object 2 (8.3%). For the 24 patients, CT pathology included epidural hematoma 3 (12.5%), subdural hematoma 12 (50.0%), intracerebral hemorrhage 14 (58.3%), subarachnoid hemorrhage 16 (66.7%), cerebral edema 8 (33.3%), midline shift 10 (41.7%), and compressed mesencephalic cisterns 10 (41.7%). An epidural hematoma, subdural hematoma, and/or intracerebral hemorrhage occurred in all 24 patients. Head Abbreviated Injury Scale scores for the early tracheostomy and the no early tracheostomy groups are in Table 1. Increased intracranial pressure rates, according to the brain CT (midline shift or compressed mesencephalic cisterns), were comparable for the early tracheostomy group and the no early tracheostomy group (See Table 1). Craniotomy rates were similar for the early tracheostomy group and the no early tracheostomy group (See Table 1). All Severity of illness comparisons for the early and no early tracheostomy groups are presented in Table 1 and show no differences between the two groups.
Figure 1.
SEHC Patient Consolidated Standards of Reporting Trials (CONSORT) Flow Chart.
Table 1.
Severity of Illness Assessment
| Study | Trait | E.T. | No E.T. | P-value |
|---|---|---|---|---|
| SEHC | Age | 33 ± 13 | 37 ± 16 | 0.44 |
| Injury Severity Score | 28 ± 11 | 35 ± 9 | 0.12 | |
| Glasgow Coma Score | 4 ± 2.5 | 4 ± 0.9 | 0.38 | |
| Head AIS | 4.7 ± 0.6 | 4.9 ± 0.3 | 0.48 | |
| Increased ICP | 6/15 (40.0%) | 5/9 (55.6%) | 0.68 | |
| Craniotomy | 7/15 (46.7%) | 4/9 (44.4%) | 0.70 | |
| Bouderka | SAPS | 5.4 ± 1.5 | 6 ± 3.8 | 0.52 |
| Age | 41 ± 18 | 40 ± 19 | 0.53 | |
| Sugerman | APACHE III Score | 65 ± 5 | 51 ± 4 | < 0.05 |
| Injury Severity Score | 30 ± 2 | 32 ± 2 | > 0.05 | |
| Glasgow Coma Score | 7 ± 0.6 | 7 ± 0.5 | > 0.05 |
E.T., early tracheostomy; SEHC, St. Elizabeth Health Center; AIS, Abbreviated Injury Score; Increased ICP, increased intracranial pressure by computed tomography (midline shift or compressed mesencephalic cisterns); SAPS, Simplified Acute Physiology Score; APACHE III Score, Acute Physiology and Chronic Health Evaluation III Score.
Suspected VAP was prospectively documented in 19 patients. Each of the 19 patients underwent diagnostic bronchoalveolar lavage and was given empiric antimicrobials. Criteria for suspected VAP in the 19 patients was chest x-ray infiltrate 16 (84.2%), P/F O2 ≤ 240 11 (57.9%), purulent tracheal secretions 10 (52.6%), abnormal WBC count 15 (78.9%), and abnormal temperature 8 (42.1%). Bronchoalveloar lavage was positive, indicating a diagnosis of VAP, in 11 of 19 (57.9%), or 11/24 (45.8%) patients. VAP rate comparisons for the early and no early tracheostomy groups are shown in Table 2. Ventilator day comparisons for the early and no early tracheostomy groups are shown in Table 3. No deaths were seen in the SEHC study.
Table 2.
Ventilator Associated Pneumonia Rates
| Study | E.T. # | VAP | No E.T. # | VAP | P-value |
|---|---|---|---|---|---|
| Sugerman | 35 | 17 (48.6%) | 32 | 17 (53.1%) | 0.71 |
| Bouderka | 31 | 18 (58.1%) | 31 | 19 (61.3%) | 0.79 |
| SEHC | 15 | 7 (46.7%) | 9 | 4 (44.4%) | 0.69 |
| Total | 81 | 42 (51.9%) | 72 | 40 (55.6%) | 0.66 |
E.T., early tracheostomy; VAP, ventilator associated pneumonia; SEHC, St. Elizabeth Health Center.
Table 3.
Ventilator/ICU Days
| Study | Day Type | E.T. # | Days | No E.T. # | Days | P-value |
|---|---|---|---|---|---|---|
| Sugerman | ICU | 35 | 16 ± 5.9 | 9 ± 11.3 | 32 | > 0.05 |
| Bouderka | ventilator | 31 | 14.5 ± 7.3 | 31 | 17.5 ± 10.6 | 0.02 |
| SEHC | ventilator | 15 | 14.1 ± 5.7 | 9 | 17.0 ± 5.4 | 0.23 |
ICU, intensive care unit; E.T., early tracheostomy; VAP, ventilator associated pneumonia; SEHC, St. Elizabeth Health Center.
Historic randomized trials
Severity of illness comparisons for the early and no early tracheostomy groups in the Sugerman and Bouderka studies are presented in Table 1. The data indicates that the early and no early groups are comparable. VAP rate results for the early and no early tracheostomy groups in the Sugerman and Bouderka studies are shown in Table 2. The Bouderka study provided ventilator day results for the early and no early groups. The Sugerman publication presented ICU days, but not ventilator days. Ventilator or ICU day comparisons for the early and no early tracheostomy groups in the Sugerman and Bouderka studies are shown in Table 3. Hospital mortality rates for the early and no early tracheostomy groups in the Sugerman and Bouderka studies are shown in Table 4.
Table 4.
Hospital Mortality Rates – Randomized Controlled Trials
| Study | E.T. # | Mortality | No E.T. # | Mortality | P-value |
|---|---|---|---|---|---|
| Sugerman | 35 | 5 (14.3%) | 32 | 1 (3.2%) | 0.20 |
| Bouderka | 31 | 12 (38.7%) | 31 | 7 (22.6%) | 0.27 |
| SEHC | 15 | 0 (0.0%) | 9 | 0 (0.0%) | > 0.05 |
| Total | 81 | 17 (21.0%) | 72 | 8 (11.1%) |
E.T., early tracheostomy; SEHC, St. Elizabeth Health Center.
Aggregate analysis of randomized trials
VAP rate comparisons for the early and no early tracheostomy groups in the three studies are shown in Table 2. VAP rates are similar for the early and no early tracheostomy groups in all three studies. Meta-analysis results are displayed in Figure 2 and do not show an aggregate difference in VAP rates according to timing of tracheostomy.
Figure 2.
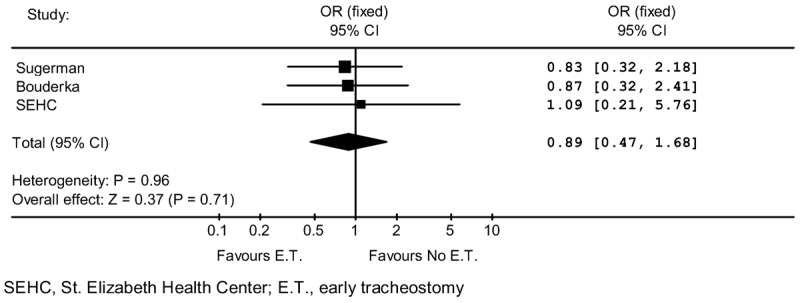
Ventilator Associated Pneumonia Rate Meta-Analysis.
Ventilator or ICU day comparisons for the early and no early tracheostomy groups in the three randomized studies are shown in Table 3. There is a three-day reduction for early tracheostomy in each of the studies. Meta-analysis results are displayed in Figure 3 and show an aggregate benefit for early tracheostomy (p = 0.02).
Figure 3.
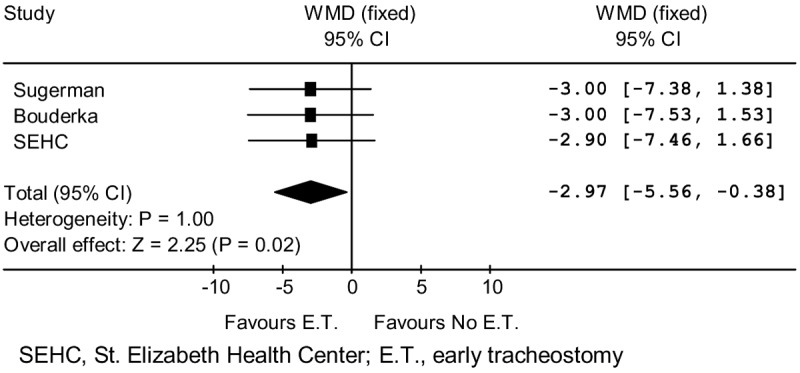
Ventilator/ICU Days Meta-Analysis.
Hospital mortality rate comparisons for the early and no early tracheostomy groups in the three randomized studies are shown in Table 4. No deaths were seen in the SEHC study. Deaths were insignificantly lower with no early tracheostomy in both the Sugerman and Bouderka studies. When all patient results were summed for the three studies, mortality was 21.0% with early tracheostomy and 11.1% with no early tracheostomy. The meta-analysis is shown in Figure 4. This process excluded SEHC patients with no deaths, because an odds ratio could not be computed. The meta-analysis indicates that there is increased hospital mortality with early tracheostomy (OR 2.7; p = 0.05).
Figure 4.
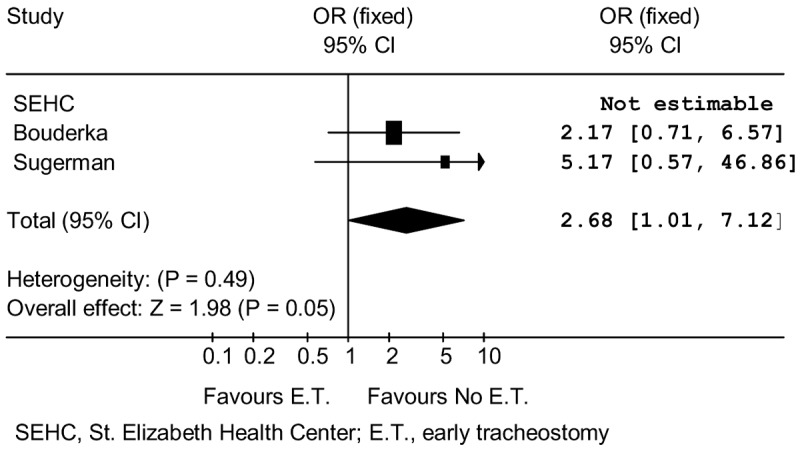
Mortality Meta-Analysis – Randomized Controlled Trials.
Retrospective study hospital mortality analysis
The literature search identified a total of five relevant retrospective studies: Ahmed, 2007 [4], Chintamani, 2005 [5], D’Amelio [6], Rizk, 2011 [7], and Wang, 2012 [8]. Retrospective study hospital mortality rate comparisons for the early and late tracheostomy groups in each study are shown in Table 5. When all patient results were summed for the five studies, mortality was 15.6% with early tracheostomy and 8.8% with late tracheostomy. The meta-analysis, shown in Figure 5, indicates increased hospital mortality with early tracheostomy (OR 1.97; p < 0.0001).
Table 5.
Hospital Mortality Rates – Retrospective Studies
| Study | E.T. # | Mortality | L.T. # | Mortality | P-value |
|---|---|---|---|---|---|
| Ahmed | 27 | 4 (14.8%) | 28 | 1 (3.6%) | 0.19 |
| Chintamani | 50 | 18 (36.0%) | 50 | 29 (58.0%) | 0.03 |
| D’Amelio | 21 | 2 (9.5%) | 10 | 1 (10.0%) | 0.70 |
| Rizk | 1,577 | 238 (15.1%) | 1,527 | 111 (7.3%) | < 0.0001 |
| Wang | 16 | 2 (12.5%) | 50 | 4 (8.0%) | 0.63 |
| Total | 1,691 | 264 (15.6%) | 1,665 | 146 (8.8%) |
E.T., early tracheostomy; L.T., late tracheostomy.
Figure 5.
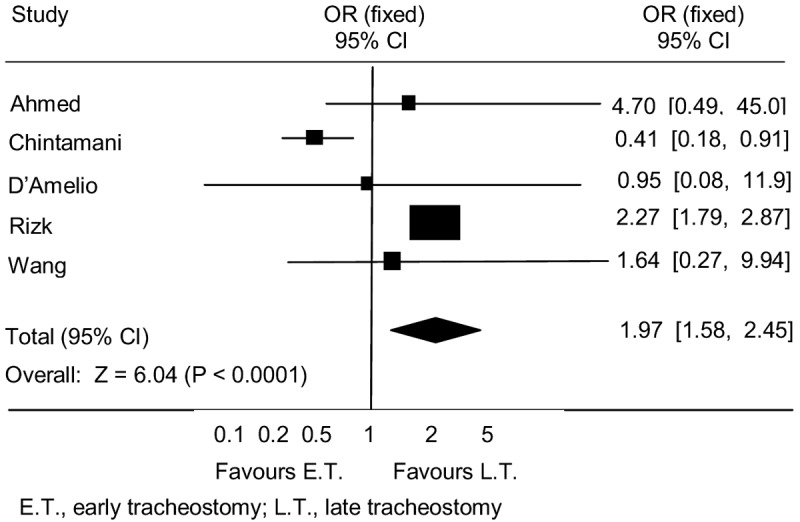
Mortality Meta-Analysis – Retrospective Studies.
Discussion
Randomized control trial methodologies
All three randomized controlled trials required patients to have a GCS ≤ 8. In addition, the SEHC and Bouderka studies required the presence of intracranial hemorrhage. The Sugerman study required at least three days of ventilation and anticipated need for an additional seven days. Thus, these findings indicate that each study contained patients with severe brain injury.
Early tracheostomy was performed 3-5 days post-injury in the SEHC and Sugerman studies. Similarly, Bouderka provided early tracheostomy on post-injury day five or six. Our previous literature review showed that early tracheostomy has typically been performed at 3-8 days following injury [1].
For the diagnosis of VAP, all three studies required clinical criteria for suspicion of VAP. The diagnosis was confirmed by bronchoalveolar lavage cultures in the SEHC study and by blind tracheal aspirate cultures in the Sugerman and Bouderka studies. Although culture methodologies for VAP diagnosis varied, recent publications indicate that practical outcomes are similar [9,10].
Only the SEHC study provided the age of the patients, with no difference between the early and no early tracheostomy groups. Severity of illness criteria varied among the three studies, however, the early and no early tracheostomy groups in each study are similar. Thus, we can conclude that outcomes in the early and no early tracheostomy groups were not related to variance in severity of illness.
Randomized control trial VAP analysis
VAP rates are virtually identical in all six randomized controlled trial patient groups. That is, VAP rates are very similar for each of the three randomized control trials in both the early tracheostomy and the no early tracheostomy groups. This indicates that the patient cohorts and interventions in the three randomized control trials were clinically homogenous. The data results are compelling that the timing of tracheostomy does not alter VAP rates in severe brain injured patients. Obviously, the meta-analysis supports this premise. Although the VAP rates are substantial, the rates are not inconsistent with other publications regarding patients with severe brain injury [11,12]. Including the Bouderka and Sugerman studies, there are five trauma-patient randomized controlled trials demonstrating no decrement in VAP rates with early tracheostomy [1].
Randomized control trial ventilator/ICU day analysis
The ventilator or ICU duration is three fewer days with early tracheostomy in each of the three randomized controlled trials. However, only in the Bouderka study does this reach statistical significance. The same three day reduction in all three randomized control trials implies that patient cohorts and interventions were clinically similar. After combining the data, the meta-analysis results indicate that ventilator or ICU days are significantly fewer with early tracheostomy. Although there is commonly a difference between ICU days and ventilator days, the meta-analysis examined the effect size between the early and no early tracheostomy groups in each of the studies. The individual study analyses and aggregate evidence seems convincing that faster liberation from the ventilator and ICU environment are related to early tracheostomy in severe brain injured patients. It is germane that an unconscious patient commonly has no need for mechanical ventilation once the airway is protected by a tracheostomy. This may be a principal reason for earlier ICU/ventilator liberation in the early tracheostomy groups. Of interest, ventilator/ICU days have been found to be similar for early tracheostomy and no early tracheostomy in three randomized trials of trauma patients, without a focus on brain injury [1].
Hospital mortality analysis
The raw sum hospital mortality rate was higher with early tracheostomy in the three randomized trials. The lack of deaths in the SEHC study was somewhat unexpected and precluded study inclusion in the meta-analysis for hospital mortality. However, it is worth noting that the no early tracheostomy mortality in the Sugerman study was only 3.2%. Mortality was insignificantly higher with early tracheostomy in both the Sugerman and Bouderka studies. However, the meta-analysis shows that hospital mortality rate is greater with early tracheostomy.
Because the numbers in the Sugerman and Bouderka studies were relatively small and the SEHC study was excluded from the mortality meta-analysis, all published retrospective studies were also evaluated. The studies by Ahmed, Chintamani, D’Amelio, Rizk, and Wang each assess the association of early tracheostomy on hospital mortality. The five investigations consist of patients with severe traumatic brain injury and were published during the last eight years, except for the older D’Amelio study. The raw sum hospital mortality rate was greater with early tracheostomy. Further, the meta-analysis of the five published retrospective studies indicates that early tracheostomy is associated with a higher hospital mortality rate. This finding enhances the validity of the meta-analysis in the randomized controlled trials that mortality is increased with early tracheostomy. Although randomized controlled trials are preferable for meta-analysis, the Cochrane Collaboration has indicated that meta-analysis of retrospective studies may be of potential value [13]. That is, use of retrospective studies may be appropriate when randomized trials are non-existent, small, or equivocal.
Using data from the Pennsylvania Trauma Foundation, the literature contains another relevant, retrospective study, by Schauer, describing severe brain injury trauma patients (GCS ≤ 8) undergoing emergency tracheal intubation and subsequent tracheostomy [14]. This study describes patients receiving tracheostomy on day 0-3, day 4-7, day 8-12, and after day 12. Hospital mortality for patients with a high-probability of survival was 7%, 7%, 1%, and 9%, respectively. Hospital mortality for patients with a low-probability of survival was 49%, 14%, 10%, and 8%, respectively. The data indicates that hospital mortality for high-probability of survival patients was lowest for tracheostomy on day 8-12. Further, results show that hospital mortality for low-probability of survival was lowest with tracheostomy beginning on hospital day 8. Data from this study indicates that hospital mortality was lowest when tracheostomy was performed after the first seven days following severe traumatic brain injury. According to this investigation, ventilator duration was less when tracheostomy was performed on day 8-12, when compared to after 12 days, regardless of survival probability. Thus, the study indicates that hospital mortality may be lowest with tracheostomy on day 8, yet ventilator liberation may also be facilitated. Because the Schauer and Rizk studies emanate from the same state; come from a similar data base, according to Rizk; and the study periods overlap, the Schauer data was not included in our retrospective mortality study analysis.
Adverse effects of severe brain injury tracheostomy
Several studies have demonstrated increases in intracranial pressure with early tracheostomy during acute brain injury. Increases in intracranial pressure are well known to adversely affect survival. Stocchetti in two studies and Kocaeli in another investigation have shown that intracranial pressure significantly increased during tracheostomy, despite pre-tracheostomy intracranial pressure control [15-17]. All three studies found intracranial pressure levels to commonly increase above 20 torr, a concerning level. One of the studies evaluating cerebral perfusion pressure and arterial carbon dioxide levels demonstrated changes that are known to adversely affect survival [16]. Of relevance, Reilly also showed that continuous bronchoscopy during percutaneous tracheostomy contributes to hypoventilation, hypercarbia, and respiratory acidosis, factors known to increase intracranial pressure [18]. The two Stocchetti studies specifically state that the investigators do not recommend tracheostomy with intracranial hypertension [15,16]. Stocchetti and Kocaeli advocate that during tracheostomy with acute brain injury, intracranial pressure should be closely monitored and preventive strategies should be instituted to prevent secondary insult [16,17].
The above implies that tracheostomy should not be routinely performed during the first seven days following severe traumatic brain injury, due to the concern for increased mortality. Of relevance, the literature indicates that a low probability of good outcomes for severe brain injury exists when there is an admission GCS 3-5, age > 45 years, intracranial hypertension, hypoxemia, or hypotension [19]. Based on the evidence, a tracheostomy should be considered in those patients beginning on day 8, if the patient is stable. That is, there is no intracranial hypertension, hypoxemia, or hypotension.
Limitations
Because the SEHC study was small, mortality findings may have been different with a larger cohort of patients. The strengths and limitations of meta-analysis are documented in the literature [20,21]. Specifically, a meta-analysis of several small studies may not predict the results of a single large study. We recognize the potential bias that may exist in the retrospective studies. However, the concordant results of the randomized trials and retrospective study meta-analysis imply that early tracheostomy mitigates acute survival. Our investigation used aggregate patient data, which is typical for most published meta-analyses. However, the use of individual patient data from each study might produce different meta-analysis results. Obviously, there would be more confidence in the meta-analysis of the randomized controlled trials if larger cohorts were included. Unless a multi-center randomized controlled trial is initiated in the near future, a larger patient volume experience is not likely in the imminent future.
Conclusions
The manuscript objectively summarizes the association of early tracheostomy for severe brain injury with VAP rates, duration of ICU/ventilator days, and hospital mortality. Evidence from three randomized controlled trials indicates that early tracheostomy is not associated with a reduction in VAP rates. However, ICU/ventilator days are reduced with early tracheostomy. Of concern, the randomized trials indicate that risk for hospital death is increased with early tracheostomy. Due to small randomized trial cohort sizes, retrospective studies were evaluated and also showed that hospital mortality is increased with early tracheostomy in severe brain injury. Study findings and relevant literature imply that tracheostomy during the first seven days following severe traumatic brain injury should not be a routine policy. Tracheostomy during the first post-injury week may be reasonable in select, stable patients.
Acknowledgements
The authors would like to acknowledge Barbara Hileman and Marina Hanes for assistance with editing the manuscript.
Disclosure of conflict of interest
There are no conflicts of interest to declare.
References
- 1.Dunham CM, Ransom KJ. Assessment of early tracheostomy in trauma patients: a systematic review and meta-analysis. Am Surg. 2006;72:276–281. doi: 10.1177/000313480607200316. [DOI] [PubMed] [Google Scholar]
- 2.Sugerman HJ, Wolfe L, Pasquale MD, Rogers FB, O’Malley KF, Knudson M, DiNardo L, Gordon M, Schaffer S. Multicenter, randomized, prospective trial of early tracheostomy. J Trauma. 1997;43:741–747. doi: 10.1097/00005373-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bouderka MA, Fakhir B, Bouaggad A, Hmamouchi B, Hamoudi D, Harti A. Early tracheostomy versus prolonged endotracheal intubation in severe head injury. J Trauma. 2004;57:251–254. doi: 10.1097/01.ta.0000087646.68382.9a. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed N, Kuo YH. Early versus late tracheostomy in patients with severe traumatic head injury. Surg Infect (Larchmt) 2007;8:343–347. doi: 10.1089/sur.2006.065. [DOI] [PubMed] [Google Scholar]
- 5.Chintamani , Khanna J, Singh JP, Kulshreshtha P, Kalra P, Priyambada B, Mohil RS. Early tracheostomy in closed head injuries: experience at a tertiary center in a developing country--a prospective study. BMC Emerg Med. 2005;5:8. doi: 10.1186/1471-227X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amelio LF, Hammond JS, Spain DA, Sutyak JP. Tracheostomy and percutaneous endoscopic gastrostomy in the management of the head-injured trauma patient. Am Surg. 1994;60:180–185. [PubMed] [Google Scholar]
- 7.Rizk EB, Patel AS, Stetter CM, Chinchilli VM, Cockroft KM. Impact of tracheostomy timing on outcome after severe head injury. Neurocrit Care. 2011;15:481–489. doi: 10.1007/s12028-011-9615-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang HK, Lu K, Liliang PC, Wang KW, Chen HJ, Chen TB, Liang CL. The impact of tracheostomy timing in patients with severe head injury: an observational cohort study. Injury. 2012;43:1432–1436. doi: 10.1016/j.injury.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 9.Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: diagnosis and treatment. J Crit Care. 2008;23:138–147. doi: 10.1016/j.jcrc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Rea-Neto A, Youssef NC, Tuche F, Brunkhorst F, Ranieri VM, Reinhart K, Sakr Y. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care. 2008;12:R56. doi: 10.1186/cc6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallel H, Chelly H, Bahloul M, Ksibi H, Dammak H, Chaari A, Ben Hamida C, Rekik N, Bouaziz M. The effect of ventilator-associated pneumonia on the prognosis of head trauma patients. J Trauma. 2005;59:705–710. [PubMed] [Google Scholar]
- 12.Zygun DA, Zuege DJ, Boiteau PJ, Laupland KB, Henderson EA, Kortbeek JB, Doig CJ. Ventilator-associated pneumonia in severe traumatic brain injury. Neurocrit Care. 2006;5:108–114. doi: 10.1385/ncc:5:2:108. [DOI] [PubMed] [Google Scholar]
- 13.Reeves BCDJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Intervention. Version 5.0.1 [updated September 2008] The Cochrane Collaboration; 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- 14.Schauer JM, Engle LL, Maugher DT, Cherry RA. Does acuity matter? Optimal timing of tracheostomy stratified by injury severity. J Trauma. 2009;66:220–225. doi: 10.1097/TA.0b013e31816073e3. [DOI] [PubMed] [Google Scholar]
- 15.Stocchetti N, Parma A, Songa V, Colombo A, Lamperti M, Tognini L. Early translaryngeal tracheostomy in patients with severe brain damage. Intensive Care Med. 2000;26:1101–1107. doi: 10.1007/s001340051324. [DOI] [PubMed] [Google Scholar]
- 16.Stocchetti N, Parma A, Lamperti M, Songa V, Tognini L. Neurophysiological consequences of three tracheostomy techniques: a randomized study in neurosurgical patients. J Neurosurg Anesthesiol. 2000;12:307–313. doi: 10.1097/00008506-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kocaeli H, Korfali E, Taskapilioglu O, Ozcan T. Analysis of intracranial pressure changes during early versus late percutaneous tracheostomy in a neuro-intensive care unit. Acta Neurochir (Wien) 2008;150:1263–1267. doi: 10.1007/s00701-008-0153-9. discussion 1267. [DOI] [PubMed] [Google Scholar]
- 18.Reilly PM, Sing RF, Giberson FA, Anderson HL 3rd, Rotondo MF, Tinkoff GH, Schwab CW. Hypercarbia during tracheostomy: a comparison of percutaneous endoscopic, percutaneous Doppler, and standard surgical tracheostomy. Intensive Care Med. 1997;23:859–864. doi: 10.1007/s001340050422. [DOI] [PubMed] [Google Scholar]
- 19.Dunham CM, Carter KJ, Castro F, Erickson B. Impact of cervical spine management brain injury on functional survival outcomes in comatose, blunt trauma patients with extremity movement and negative cervical spine CT: application of the Monte Carlo simulation. J Neurotrauma. 2011;28:1009–1019. doi: 10.1089/neu.2010.1301. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Kuderer NM. The strengths and limitations of meta-analyses based on aggregate data. BMC Med Res Methodol. 2005;5:14. doi: 10.1186/1471-2288-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker E, Hernandez AV, Kattan MW. Meta-analysis: Its strengths and limitations. Cleve Clin J Med. 2008;75:431–439. doi: 10.3949/ccjm.75.6.431. [DOI] [PubMed] [Google Scholar]