Figure 5.
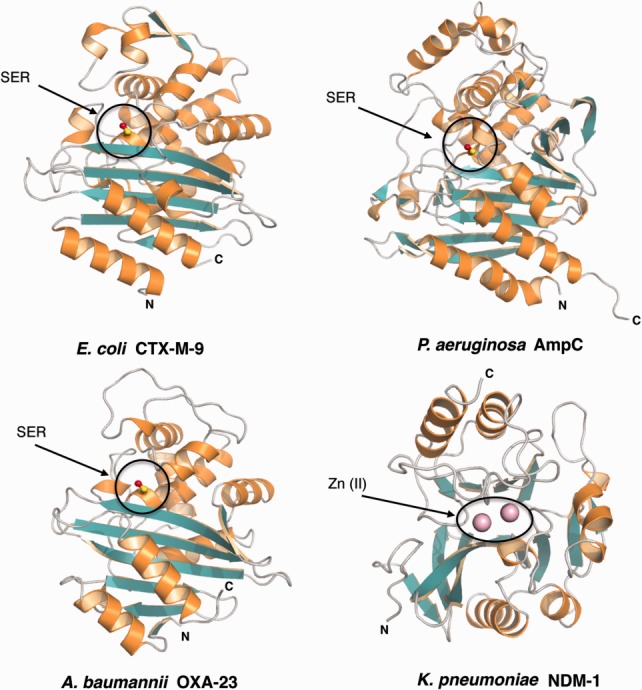
Structures of class A, C, D, and B β-lactamases. The conserved catalytic serine (SER) of classes A (CTX-M-9), C (AmpC), and D (OXA-23) β-lactamases is embedded at the interface between two closely interacting domains, shown in orange and green. NDM-1, a class B enzyme, differs from these serine-β-lactamases in fold and chemical mechanism. The enzyme shows a αβ/βα fold with an active site located at the edge of the β-sandwich. The active site is occupied by divalent zinc ions shown as pink spheres.
