Fig. 3.
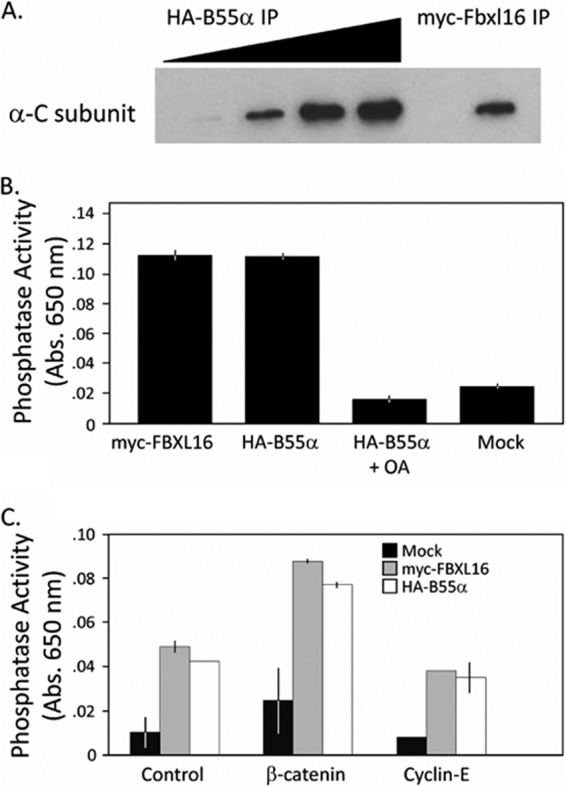
FBXL16 binding to B55α-PP2A does not alter the intrinsic catalytic activity of the enzyme. A, relative level of PP2A catalytic subunit in myc-FBXL16 and HA-B55α immunoprecipitates. Amounts of PP2A catalytic subunit C were estimated through volumetric titration of HA-B55α immunoprecipitate compared with a fixed volume of myc-FBXL16 immunoprecipitate. Samples were fractionated via SDS-PAGE and immunoblotted with antibody against the C subunit. B, robust phosphatase activity is associated with FBXL16. The specific PP2A activity was calculated based on the amount of catalytic subunit (A), and whole cell lysates from 293 cells transfected with either no vector (Mock) or myc-FBXL16 or HA-B55α expression vectors were immunoprecipitated and assayed for serine/threonine phosphatase activity using a commercial PP2A phosphopeptide assay. Error bars represent ±1 S.D.; n = 3 for each sample. OA = okadaic acid, 50 nm final concentration. C, same as B, except that phosphorylated β-catenin (a known substrate of the B55α form of PP2A) and cyclin E (not a described PP2A substrate) peptide substrates were used (37). The peptide substrates employed here are the same as those used in prior ubiquitination studies (27, 28). The commercial PP2A peptide was used as a positive control, and measurements were made in duplicate. Error bars represent ±1 S.D.
