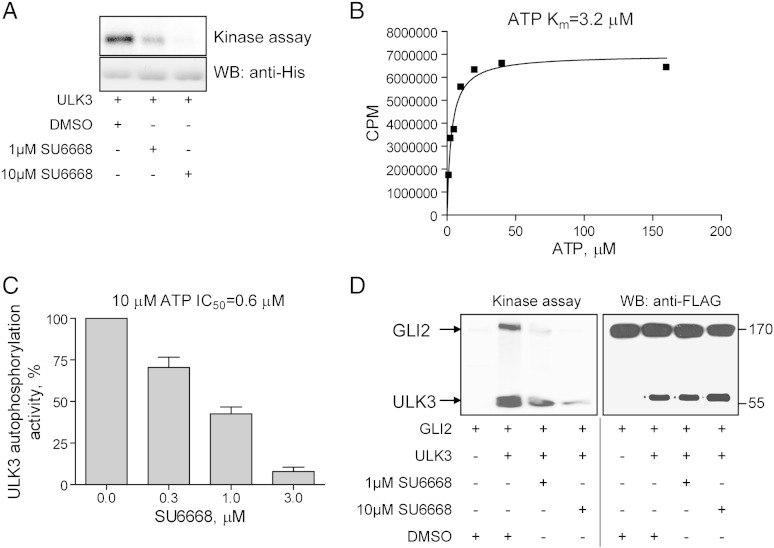
Fig. 1.
SU6668 inhibits ULK3 catalytic activity in vitro. (A) Bacterially expressed and purified His-tagged ULK3 protein was subjected to in vitro kinase assay in the presence of 2 μM ATP and vehicle or SU6668. The presence of ULK3 protein was verified by WB. (B) His-tagged ULK3 protein was subjected to in vitro kinase assay in the presence of indicated concentrations of ATP. The Michaelis–Menten curve was built using levels of ULK3 autophosphorylation activity normalized with amount of ULK3 protein identified by Coomassie staining (both quantified using ImageQuant software). (C) ULK3 protein was subjected to in vitro kinase assay in the presence of 10 μM ATP. Different concentrations of SU6668 were included into each reaction. The intensity of ULK3 autophosphorylation and the amount of ULK3 protein identified by Coomassie staining were quantified using ImageQuant software. The normalized level of ULK3 autophosphorylation activity in the presence of vehicle was set as 100%. (D) GLI2 and ULK3 were over-expressed and immuno-purified using a resin conjugated with FLAG antibody. The purified proteins were mixed and subjected to in vitro kinase assay (left panel). The proteins were verified by WB (right panel).