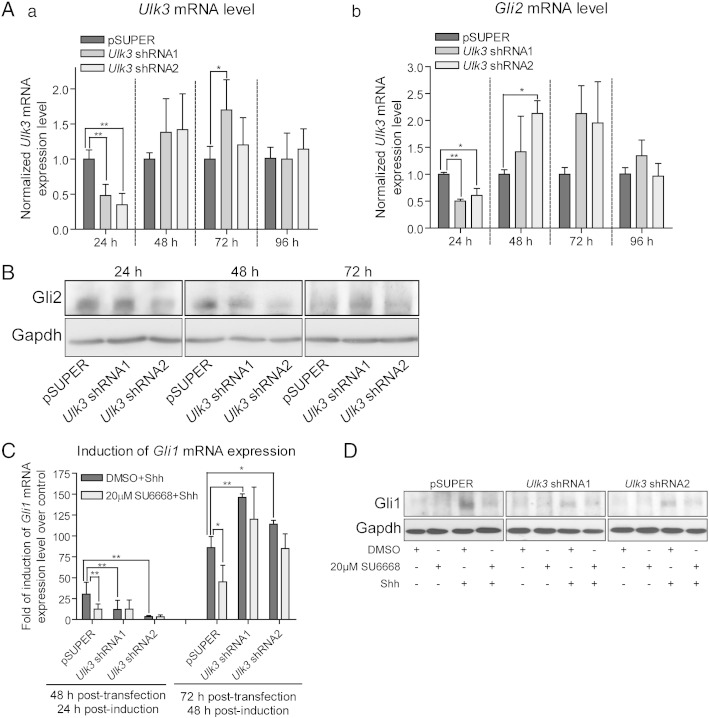
Supplementary Fig. 3.
(A) C3H10T1/2 cells were transfected with empty vector pSUPER or Ulk3 shRNAs and incubated during the indicated periods of time. Expression levels of Ulk3 (a) and Gli2 (b) were analyzed using qRT-PCR. The normalized with Hprt level of the respective gene expression at the indicated time point was set as 1 in the control cells transfected with pSUPER, and the data from the samples transfected with Ulk3 shRNAs were calculated relative to the control. The data are presented as an average mean ± S.D. (B) Expression of Gli2 and GAPDH proteins was analyzed by Western-blotting at the indicated time points in the post-transfectional C3H10T1/2 cells electroporated with empty vector pSUPER or Ulk3 shRNAs. Gli2 was detected using AF3635 antibody. (C) C3H10T1/2 cells were electroporated with pSUPER or Ulk3 shRNAs encoding constructs, incubated for 24 h and treated with DMSO or SU6668 in the presence or absence of Shh for additional 24 h or 48 h. Gli1 mRNA expression level was measured by qRT-PCR after 24 h and 48 h of treatment initiation (after 48 h and 72 h of transfection, respectively). For each data set obtained from the cells transfected with the same construct, the level of Gli1 mRNA expression was accepted as 1 in the control samples treated with DMSO. Data from other samples were calculated relative to their respectively transfected controls. The data are presented as an average mean of induction of Gli1 mRNA expression over control ± S.D. (D) C3H10T1/2 cells were transfected with pSUPER or Ulk3 shRNAs encoding constructs, incubated for 24 h and treated as indicated for additional 48 h. Levels of Gli1 and GAPDH proteins were examined by immuno-blotting (48 h post-treatment, 72 h post-transfection); *—p < 0.05, **—p < 0.01, n = 3.